Semi-Biosynthetic Production of Surface-Binding Adhesive Antimicrobial Peptides Using Intein-Mediated Protein Ligation
- PMID: 36499519
- PMCID: PMC9738365
- DOI: 10.3390/ijms232315202
Semi-Biosynthetic Production of Surface-Binding Adhesive Antimicrobial Peptides Using Intein-Mediated Protein Ligation
Abstract
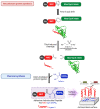
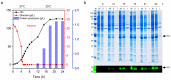
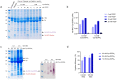
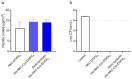
Microbial infections remain a global health concern, calling for the urgent need to implement effective prevention measures. Antimicrobial peptides (AMPs) have been extensively studied as potential antimicrobial coating agents. However, an efficient and economical method for AMP production is lacking. Here, we synthesized the direct coating adhesive AMP, NKC-DOPA5, composed of NKC, a potent AMP, and repeats of the adhesive amino acid 3,4-dihydroxyphenylalanine (DOPA) via an intein-mediated protein ligation strategy. NKC was expressed as a soluble fusion protein His-NKC-GyrA (HNG) in Escherichia coli, comprising an N-terminal 6× His-tag and a C-terminal Mxe GyrA intein. The HNG protein was efficiently produced in a 500-L fermenter, with a titer of 1.63 g/L. The NKC-thioester was released from the purified HNG fusion protein by thiol attack and subsequently ligated with chemically synthesized Cys-DOPA5. The ligated peptide His-NKC-Cys-DOPA5 was obtained at a yield of 88.7%. The purified His-NKC-Cys-DOPA5 possessed surface-binding and antimicrobial properties identical to those of the peptide obtained via solid-phase peptide synthesis. His-NKC-Cys-DOPA5 can be applied as a practical and functional antimicrobial coating to various materials, such as medical devices and home appliances.
Keywords: Escherichia coli; antimicrobial peptide; intein; intein-mediated protein ligation.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Adhesive Antimicrobial Peptides Containing 3,4-Dihydroxy-L-Phenylalanine Residues for Direct One-Step Surface Coating.Int J Mol Sci. 2021 Nov 3;22(21):11915. doi: 10.3390/ijms222111915. Int J Mol Sci. 2021. PMID: 34769345 Free PMC article.
-
Production of antimicrobial peptide arasin-like Sp in Escherichia coli via an ELP-intein self-cleavage system.J Biotechnol. 2022 Mar 10;347:49-55. doi: 10.1016/j.jbiotec.2022.02.010. Epub 2022 Feb 28. J Biotechnol. 2022. PMID: 35240202
-
Production of a polar fish antimicrobial peptide in Escherichia coli using an ELP-intein tag.J Biotechnol. 2016 Sep 20;234:83-89. doi: 10.1016/j.jbiotec.2016.07.021. Epub 2016 Jul 30. J Biotechnol. 2016. PMID: 27485812
-
Site-specific protein labeling by intein-mediated protein ligation.Methods Mol Biol. 2011;705:87-107. doi: 10.1007/978-1-61737-967-3_6. Methods Mol Biol. 2011. PMID: 21125382 Review.
-
Cleavable self-aggregating tags (cSAT) for protein expression and purification.Methods Mol Biol. 2015;1258:65-78. doi: 10.1007/978-1-4939-2205-5_4. Methods Mol Biol. 2015. PMID: 25447859 Review.
Cited by
-
Design of a recombinant asparaginyl ligase for site-specific modification using efficient recognition and nucleophile motifs.Commun Chem. 2024 Apr 18;7(1):87. doi: 10.1038/s42004-024-01173-8. Commun Chem. 2024. PMID: 38637620 Free PMC article.
-
Anti-Staphy Peptides Rationally Designed from Cry10Aa Bacterial Protein.ACS Omega. 2024 Jun 19;9(27):29159-29174. doi: 10.1021/acsomega.3c07455. eCollection 2024 Jul 9. ACS Omega. 2024. PMID: 39005792 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

