The Role of Epigenetics in Neuroinflammatory-Driven Diseases
- PMID: 36499544
- PMCID: PMC9740629
- DOI: 10.3390/ijms232315218
The Role of Epigenetics in Neuroinflammatory-Driven Diseases
Abstract
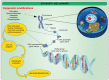
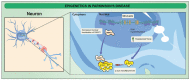
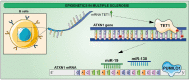
Neurodegenerative disorders are characterized by the progressive loss of central and/or peripheral nervous system neurons. Within this context, neuroinflammation comes up as one of the main factors linked to neurodegeneration progression. In fact, neuroinflammation has been recognized as an outstanding factor for Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS), Parkinson's disease (PD), and multiple sclerosis (MS). Interestingly, neuroinflammatory diseases are characterized by dramatic changes in the epigenetic profile, which might provide novel prognostic and therapeutic factors towards neuroinflammatory treatment. Deep changes in DNA and histone methylation, along with histone acetylation and altered non-coding RNA expression, have been reported at the onset of inflammatory diseases. The aim of this work is to review the current knowledge on this field.
Keywords: Alzheimer’s disease; Parkinson’s disease; amyotrophic lateral sclerosis; chronic neuroinflammation; epigenetics; multiple sclerosis; neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Histone Methylation Regulation in Neurodegenerative Disorders.Int J Mol Sci. 2021 Apr 28;22(9):4654. doi: 10.3390/ijms22094654. Int J Mol Sci. 2021. PMID: 33925016 Free PMC article. Review.
-
Neurodegenerative diseases: Epigenetic regulatory mechanisms and therapeutic potential.Cell Signal. 2025 Jul;131:111715. doi: 10.1016/j.cellsig.2025.111715. Epub 2025 Mar 13. Cell Signal. 2025. PMID: 40089090 Review.
-
Targeting epigenetics as a promising therapeutic strategy for treatment of neurodegenerative diseases.Biochem Pharmacol. 2022 Dec;206:115295. doi: 10.1016/j.bcp.2022.115295. Epub 2022 Oct 12. Biochem Pharmacol. 2022. PMID: 36241096 Review.
-
Epigenetic Explorations of Neurological Disorders, the Identification Methods, and Therapeutic Avenues.Int J Mol Sci. 2024 Oct 30;25(21):11658. doi: 10.3390/ijms252111658. Int J Mol Sci. 2024. PMID: 39519209 Free PMC article. Review.
-
Epigenetic Regulation of Neuroinflammation in Alzheimer's Disease.Cells. 2023 Dec 29;13(1):79. doi: 10.3390/cells13010079. Cells. 2023. PMID: 38201283 Free PMC article. Review.
Cited by
-
Unraveling the Epigenetic Landscape: Insights into Parkinson's Disease, Amyotrophic Lateral Sclerosis, and Multiple Sclerosis.Brain Sci. 2024 May 29;14(6):553. doi: 10.3390/brainsci14060553. Brain Sci. 2024. PMID: 38928553 Free PMC article. Review.
-
The Pleiotropic Effects of Fumarate: From Mitochondrial Respiration to Epigenetic Rewiring and DNA Repair Mechanisms.Metabolites. 2023 Jul 24;13(7):880. doi: 10.3390/metabo13070880. Metabolites. 2023. PMID: 37512586 Free PMC article. Review.
-
The Intersection of Epigenetics and Senolytics in Mechanisms of Aging and Therapeutic Approaches.Biomolecules. 2024 Dec 26;15(1):18. doi: 10.3390/biom15010018. Biomolecules. 2024. PMID: 39858413 Free PMC article. Review.
-
The Role of Epigenetic Mechanisms in the Development of PM2.5-Induced Cognitive Impairment.Toxics. 2025 Feb 2;13(2):119. doi: 10.3390/toxics13020119. Toxics. 2025. PMID: 39997934 Free PMC article. Review.
-
Aging exacerbates oxidative stress and liver fibrosis in an animal model of Down Syndrome.Aging (Albany NY). 2024 Jun 26;16(12):10203-10215. doi: 10.18632/aging.205970. Epub 2024 Jun 26. Aging (Albany NY). 2024. PMID: 38942607 Free PMC article.
References
-
- Ghezzi P., Bernardini R., Giuffrida R., Bellomo M., Manzoni C., Comoletti D., Di Santo E., Benigni F., Mennini T. Tumor necrosis factor is increased in the spinal cord of an animal model of motor neuron degeneration. Eur. Cytokine Netw. 1998;9:139–144. - PubMed
-
- Vicario N., Castrogiovanni P., Imbesi R., Giallongo S., Mannino G., Furno D.L., Giuffrida R., Zappala A., Li Volti G., Tibullo D., et al. GJA1/CX43 High Expression Levels in the Cervical Spinal Cord of ALS Patients Correlate to Microglia-Mediated Neuroinflammatory Profile. Biomedicines. 2022;10:2246. doi: 10.3390/biomedicines10092246. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

