Regulation of Prepro-NeuropeptideW/B and Its Receptor in the Heart of ZDF Rats: An Animal Model of Type II DM
- PMID: 36499546
- PMCID: PMC9739957
- DOI: 10.3390/ijms232315219
Regulation of Prepro-NeuropeptideW/B and Its Receptor in the Heart of ZDF Rats: An Animal Model of Type II DM
Abstract
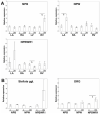
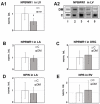
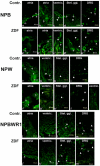
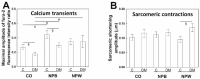
Neuropeptide B (NPB) and neuropeptide W (NPW) are neuropeptides, which constitute NPB/W signaling systems together with G-protein coupled receptors NPBWR1. The location and function of NPB/W signaling systems have been predominantly detected and mapped within the CNS, including their role in the modulation of inflammatory pain, neuroendocrine functions, and autonomic nervous systems. The aim of the study is to investigate the impact of diabetes on the neuropeptide B/W signaling system in different heart compartments and neurons which innervates it. In the RT-qPCR analysis, we observed the upregulation of mRNA for preproNPB in RV, for preproNPW in LA, and for NPBWR1 in DRG in diabetic rats. On the contrary, the expression of mRNA for NPBWR1 was downregulated in LV in diabetic rats. In the WB analysis, significant downregulation of NPBWR1 in LV (0.54-fold, p = 0.046) in diabetic rats was observed at the proteomic level. The presence of NPBWR1 was also confirmed in a dissected LCM section of cardiomyocytes and coronary arteries. The positive inotropic effect of NPW described on the diabetic cardiomyocytes in vitro could point to a possible therapeutic target for compensation of the contractile dysfunction in the diabetic heart. In conclusion, the NPB/W signaling system is involved in the regulation of heart functions and long-term diabetes leads to changes in the expression of individual members of this signaling system differently in each cardiac compartment, which is related to the different morphology and function of these cardiac chambers.
Keywords: NPBWR1; RT-qPCR; ZDF rat; calcium transients; cardiomyocytes; contraction; laser capture microdissection; neuropeptide B; neuropeptide W; western blot.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Slavíková J., Mistrová E., Dvořáková M.C. Pathophysiology of diabetic cardiomyopathy. Diabetol. Metab. Endokrinol. Vyziv. 2018;21:21–29.
-
- Dvorakova M.C., Wiegand S., Pesta M., Slavikova J., Grau V., Reischig J., Kuncova J., Kummer W. Expression of neuropeptide Y and its receptors Y1 and Y2 in the rat heart and its supplying autonomic and spinal sensory ganglia in experimentally induced diabetes. Neuroscience. 2008;151:1016–1028. doi: 10.1016/j.neuroscience.2007.07.069. - DOI - PubMed
-
- Matyal R., Mahmood F., Robich M., Glazer H., Khabbaz K., Hess P., Bianchi C., Hagberg R., Hu S.X., Sellke F.W. Chronic type II diabetes mellitus leads to changes in neuropeptide Y receptor expression and distribution in human myocardial tissue. Eur. J. Pharmacol. 2011;665:19–28. doi: 10.1016/j.ejphar.2011.04.039. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

