Effect of High Viscosity on Energy Metabolism and Kinematics of Spermatozoa from Three Mouse Species Incubated under Capacitating Conditions
- PMID: 36499575
- PMCID: PMC9737050
- DOI: 10.3390/ijms232315247
Effect of High Viscosity on Energy Metabolism and Kinematics of Spermatozoa from Three Mouse Species Incubated under Capacitating Conditions
Abstract
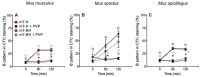
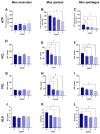
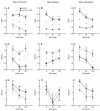
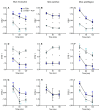
In order to sustain motility and prepare for fertilization, sperm require energy. The characterization of sperm ATP production and usage in mouse species revealed substantial differences in metabolic pathways that can be differentially affected by capacitation. Moreover, spermatozoa encounter different environments with varying viscoelastic properties in the female reproductive tract. Here, we examine whether viscosity affects sperm ATP levels and kinematics during capacitation in vitro. Sperm from three mouse species (Mus musculus, M. spretus, M. spicilegus) were incubated under capacitating conditions in a modified Tyrode's medium containing bicarbonate, glucose, pyruvate, lactate, and bovine serum albumin (mT-BH) or in a bicarbonate-free medium as a non-capacitating control. Viscosity was increased with the inclusion of polyvinylpyrrolidone. ATP was measured with a bioluminescence kit, and kinematics were examined with a computer-aided sperm analysis system. In M. musculus sperm, ATP declined during capacitation, but no differences were found between non-capacitating and capacitating sperm. In contrast, in M. spretus and M. spicilegus, ATP levels decreased in capacitating sperm. Increasing viscosity in the medium did not modify the timing or proportion of cells undergoing capacitation but did result in additional time- and concentration-dependent decreases in ATP in M. spretus and M. spicilegus under capacitating conditions. Additionally, increased viscosity altered both velocity and trajectory descriptors. The limited impact of capacitation and higher viscosity on M. musculus sperm ATP and kinematics could be related to the low intensity of postcopulatory sexual selection in this species. Responses seen in the other two species could be linked to the ability of their sperm to perform better under enhanced selective pressures.
Keywords: ATP; bioenergetics; capacitation; hyperactivation; sperm motility.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Energy Metabolism and Hyperactivation of Spermatozoa from Three Mouse Species under Capacitating Conditions.Cells. 2022 Jan 10;11(2):220. doi: 10.3390/cells11020220. Cells. 2022. PMID: 35053337 Free PMC article.
-
Effect of bicarbonate and polyvinyl alcohol on in vitro capacitation and fertilization ability of cryopreserved equine spermatozoa.Andrology. 2025 Feb;13(2):382-395. doi: 10.1111/andr.13667. Epub 2024 May 28. Andrology. 2025. PMID: 38804843
-
Bioenergetic changes in response to sperm capacitation and two-way metabolic compensation in a new murine model.Cell Mol Life Sci. 2022 Dec 19;80(1):11. doi: 10.1007/s00018-022-04652-0. Cell Mol Life Sci. 2022. PMID: 36534181 Free PMC article.
-
Bioenergetics of fish spermatozoa during semen storage.Fish Physiol Biochem. 2009 Nov;35(4):607-14. doi: 10.1007/s10695-009-9308-8. Epub 2009 Feb 27. Fish Physiol Biochem. 2009. PMID: 19247796 Review.
-
The other side of capacitation: role of mouse male molecules in the regulation of time and place of capacitation.Reproduction. 2023 Nov 10;166(6):R73-R85. doi: 10.1530/REP-23-0188. Print 2023 Dec 1. Reproduction. 2023. PMID: 37796747 Review.
Cited by
-
Bioenergetics of Rodent Spermatozoa.Methods Mol Biol. 2025;2897:267-288. doi: 10.1007/978-1-0716-4406-5_19. Methods Mol Biol. 2025. PMID: 40202642
-
The Known and Unknown About Female Reproductive Tract Mucus Rheological Properties.Bioessays. 2025 Jun;47(6):e70002. doi: 10.1002/bies.70002. Epub 2025 Mar 22. Bioessays. 2025. PMID: 40119784 Free PMC article. Review.
-
Effect of Probiotics on Sperm Quality in the Adult Mouse.Probiotics Antimicrob Proteins. 2024 Oct 23. doi: 10.1007/s12602-024-10388-z. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 39441338
-
Scriptaid is a prospective agent for improving human asthenozoospermic sample quality and fertilization rate in vitro.Asian J Androl. 2024 Sep 1;26(5):490-499. doi: 10.4103/aja202416. Epub 2024 Jun 11. Asian J Androl. 2024. PMID: 38856299 Free PMC article.
References
-
- Nixon B., Bromfield E.G. Sperm capacitation. In: Skinner M.K., editor. Encyclopedia of Reproduction. Academic Press; Oxford, UK: 2018. pp. 272–278.
-
- Yanagimachi R. Mechanisms of fertilization in mammals. In: Mastroiani L., Biggers J.D., editors. Fertilization and Embryonic Development In Vitro. Springer; Boston, MA, USA: 1981. pp. 81–182.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

