Osteopontin Splicing Isoforms Contribute to Endometriotic Proliferation, Migration, and Epithelial-Mesenchymal Transition in Endometrial Epithelial Cells
- PMID: 36499654
- PMCID: PMC9738877
- DOI: 10.3390/ijms232315328
Osteopontin Splicing Isoforms Contribute to Endometriotic Proliferation, Migration, and Epithelial-Mesenchymal Transition in Endometrial Epithelial Cells
Abstract
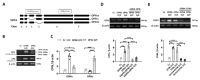
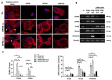
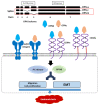
Osteopontin (OPN) isoforms, including OPNb and OPNc, promote malignancy and may contribute to the pathogenesis of endometriosis, a benign disorder with multiple characteristics resembling malignant tumors. In our experiments, OPNb and OPNc were significantly overexpressed in both endometriosis and adenomyosis compared to the normal endometrium. Upregulation of CD44v and the epithelial-mesenchymal transition (EMT) process was also present in endometriotic lesions. Overexpression of OPNb and OPNc splicing variants in endometriotic cells evoked morphological changes, actin remodeling, cell proliferation, cell migration, and EMT through binding OPN ligand receptors CD44 and αvβ3, subsequently activating the PI3K and NF-ĸB pathways. We elucidated the causal role of OPN splice variants in regulating endometriotic cell growth, which may promote the development of OPN-targeted therapies for patients suffering from endometriotic disorders.
Keywords: CD44; endometriosis; epithelial-mesenchymal transition; migration; osteopontin splicing variants; proliferation; αvβ3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Osteopontin splice variants expression is involved on docetaxel resistance in PC3 prostate cancer cells.Tumour Biol. 2016 Feb;37(2):2655-63. doi: 10.1007/s13277-015-4095-6. Epub 2015 Sep 24. Tumour Biol. 2016. PMID: 26404131
-
Both osteopontin-c and osteopontin-b splicing isoforms exert pro-tumorigenic roles in prostate cancer cells.Prostate. 2012 Nov;72(15):1688-99. doi: 10.1002/pros.22523. Epub 2012 Apr 11. Prostate. 2012. PMID: 22495819
-
Functional heterogeneity of osteopontin isoforms in non-small cell lung cancer.J Thorac Oncol. 2010 Oct;5(10):1516-23. doi: 10.1097/JTO.0b013e3181eba6bd. J Thorac Oncol. 2010. PMID: 20689445 Free PMC article.
-
Cancer-associated mutations in endometriosis: shedding light on the pathogenesis and pathophysiology.Hum Reprod Update. 2020 Apr 15;26(3):423-449. doi: 10.1093/humupd/dmz047. Hum Reprod Update. 2020. PMID: 32154564 Review.
-
Osteopontin b and c Splice isoforms in Leukemias and Solid Tumors: Angiogenesis Alongside Chemoresistance.Asian Pac J Cancer Prev. 2018 Mar 27;19(3):615-623. doi: 10.22034/APJCP.2018.19.3.615. Asian Pac J Cancer Prev. 2018. PMID: 29580029 Free PMC article. Review.
Cited by
-
Immunoregulatory Roles of Osteopontin in Diseases.Nutrients. 2024 Jan 20;16(2):312. doi: 10.3390/nu16020312. Nutrients. 2024. PMID: 38276550 Free PMC article. Review.
-
The immune duality of osteopontin and its therapeutic implications for kidney transplantation.Front Immunol. 2025 Feb 28;16:1520777. doi: 10.3389/fimmu.2025.1520777. eCollection 2025. Front Immunol. 2025. PMID: 40093009 Free PMC article. Review.
-
SPP1 promotes tumor progression in esophageal carcinoma by activating focal adhesion pathway.J Gastrointest Oncol. 2024 Jun 30;15(3):818-828. doi: 10.21037/jgo-24-302. Epub 2024 Jun 27. J Gastrointest Oncol. 2024. PMID: 38989403 Free PMC article.
-
Epithelial-mesenchymal transition is associated with osteopontin-induced EGFR‑TKI resistance in EGFR mutant non-small cell lung cancer.J Thorac Dis. 2023 Jun 30;15(6):3359-3371. doi: 10.21037/jtd-23-818. Epub 2023 Jun 26. J Thorac Dis. 2023. PMID: 37426126 Free PMC article.
-
Osteopontin as a Biomarker in Chronic Kidney Disease.Biomedicines. 2023 May 4;11(5):1356. doi: 10.3390/biomedicines11051356. Biomedicines. 2023. PMID: 37239027 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

