Revisiting the Anti-Cancer Toxicity of Clinically Approved Platinating Derivatives
- PMID: 36499737
- PMCID: PMC9793759
- DOI: 10.3390/ijms232315410
Revisiting the Anti-Cancer Toxicity of Clinically Approved Platinating Derivatives
Abstract
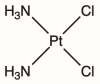
Cisplatin (CDDP), carboplatin (CP), and oxaliplatin (OXP) are three platinating agents clinically approved worldwide for use against a variety of cancers. They are canonically known as DNA damage inducers; however, that is only one of their mechanisms of cytotoxicity. CDDP mediates its effects through DNA damage-induced transcription inhibition and apoptotic signalling. In addition, CDDP targets the endoplasmic reticulum (ER) to induce ER stress, the mitochondria via mitochondrial DNA damage leading to ROS production, and the plasma membrane and cytoskeletal components. CP acts in a similar fashion to CDDP by inducing DNA damage, mitochondrial damage, and ER stress. Additionally, CP is also able to upregulate micro-RNA activity, enhancing intrinsic apoptosis. OXP, on the other hand, at first induces damage to all the same targets as CDDP and CP, yet it is also capable of inducing immunogenic cell death via ER stress and can decrease ribosome biogenesis through its nucleolar effects. In this comprehensive review, we provide detailed mechanisms of action for the three platinating agents, going beyond their nuclear effects to include their cytoplasmic impact within cancer cells. In addition, we cover their current clinical use and limitations, including side effects and mechanisms of resistance.
Keywords: DNA damage; ER stress response; carboplatin; cellular uptake; chemoresistance; cisplatin; clinical usages; immunogenic cell death; influx and efflux pumps; interstrand and intrastrand DNA crosslinks; mechanisms of action; non-nuclear targets; oxaliplatin; ribosome biogenesis; transcription regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

