Chromate-Free Corrosion Protection Strategies for Magnesium Alloys-A Review: Part III-Corrosion Inhibitors and Combining Them with Other Protection Strategies
- PMID: 36499985
- PMCID: PMC9736638
- DOI: 10.3390/ma15238489
Chromate-Free Corrosion Protection Strategies for Magnesium Alloys-A Review: Part III-Corrosion Inhibitors and Combining Them with Other Protection Strategies
Abstract
Owing to the unique active corrosion protection characteristic of hexavalent chromium-based systems, they have been projected to be highly effective solutions against the corrosion of many engineering metals. However, hexavalent chromium, rendered a highly toxic and carcinogenic substance, is being phased out of industrial applications. Thus, over the past few years, extensive and concerted efforts have been made to develop environmentally friendly alternative technologies with comparable or better corrosion protection performance to that of hexavalent chromium-based technologies. The introduction of corrosion inhibitors to a coating system on magnesium surface is a cost-effective approach not only for improving the overall corrosion protection performance, but also for imparting active inhibition during the service life of the magnesium part. Therefore, in an attempt to resemble the unique active corrosion protection characteristic of the hexavalent chromium-based systems, the incorporation of inhibitors to barrier coatings on magnesium alloys has been extensively investigated. In Part III of the Review, several types of corrosion inhibitors for magnesium and its alloys are reviewed. A discussion of the state-of-the-art inhibitor systems, such as iron-binding inhibitors and inhibitor mixtures, is presented, and perspective directions of research are outlined, including in silico or computational screening of corrosion inhibitors. Finally, the combination of corrosion inhibitors with other corrosion protection strategies is reviewed. Several reported highly protective coatings with active inhibition capabilities stemming from the on-demand activation of incorporated inhibitors can be considered a promising replacement for hexavalent chromium-based technologies, as long as their deployment is adequately addressed.
Keywords: corrosion; hexavalent chromium; inhibitor; magnesium; self-healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

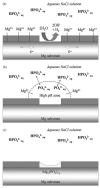

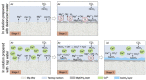



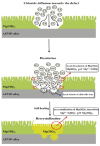
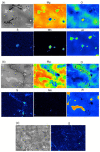





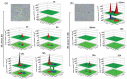

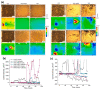
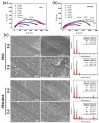
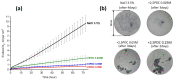
References
-
- Handbook A. Corrosion–Fundamentals, Testing and Protection. Volume 13A ASM International, The Materials Information Society; Materials Park, OH, USA: 2003.
-
- Flick E.W. Corrosion Inhibitors: An Industrial Guide. Noyes Publications; Park Ridge, NJ, USA: 1987.
-
- Sastri V.S. Green Corrosion Inhibitors: Theory and Practice. Wiley; Hoboken, NJ, USA: 2012.
-
- ECHA Authorisation List. [(accessed on 1 September 2017)];2017 Available online: https://echa.europa.eu/authorisation-list.
-
- ECHA Authorisation List with Sunset Dates. [(accessed on 1 September 2017)];2017 Available online: https://echa.europa.eu/authorisation-list?p_p_id=disslists_WAR_disslists....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

