Chromate-Free Corrosion Protection Strategies for Magnesium Alloys-A Review: Part II-PEO and Anodizing
- PMID: 36500010
- PMCID: PMC9737229
- DOI: 10.3390/ma15238515
Chromate-Free Corrosion Protection Strategies for Magnesium Alloys-A Review: Part II-PEO and Anodizing
Abstract
Although hexavalent chromium-based protection systems are effective and their long-term performance is well understood, they can no longer be used due to their proven Cr(VI) toxicity and carcinogenic effect. The search for alternative protection technologies for Mg alloys has been going on for at least a couple of decades. However, surface treatment systems with equivalent efficacies to that of Cr(VI)-based ones have only begun to emerge much more recently. It is still proving challenging to find sufficiently protective replacements for Cr(VI) that do not give rise to safety concerns related to corrosion, especially in terms of fulfilling the requirements of the transportation industry. Additionally, in overcoming these obstacles, the advantages of newly introduced technologies have to include not only health safety but also need to be balanced against their added cost, as well as being environmentally friendly and simple to implement and maintain. Anodizing, especially when carried out above the breakdown potential (technology known as Plasma Electrolytic Oxidation (PEO)) is an electrochemical oxidation process which has been recognized as one of the most effective methods to significantly improve the corrosion resistance of Mg and its alloys by forming a protective ceramic-like layer on their surface that isolates the base material from aggressive environmental agents. Part II of this review summarizes developments in and future outlooks for Mg anodizing, including traditional chromium-based processes and newly developed chromium-free alternatives, such as PEO technology and the use of organic electrolytes. This work provides an overview of processing parameters such as electrolyte composition and additives, voltage/current regimes, and post-treatment sealing strategies that influence the corrosion performance of the coatings. This large variability of the fabrication conditions makes it possible to obtain Cr-free products that meet the industrial requirements for performance, as expected from traditional Cr-based technologies.
Keywords: Cr(VI)-based coatings; coating; magnesium; micro-arc oxidation (MAO).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
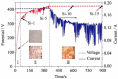
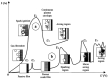




















References
-
- Esmaily M., Svensson J.E., Fajardo S., Birbilis N., Frankel G.S., Virtanen S., Arrabal R., Thomas S., Johansson L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017;89:92–193. doi: 10.1016/j.pmatsci.2017.04.011. - DOI
-
- Atrens A., Johnston S., Shi Z., Dargusch M.S. Viewpoint—Understanding Mg corrosion in the body for biodegradable medical implants. Scr. Mater. 2018;154:92–100. doi: 10.1016/j.scriptamat.2018.05.021. - DOI
-
- Vaghefinazari B., Wierzbicka E., Visser P., Posner R., Jordens G., Arrabal R., Matykina E., Mohedano M., Blawert C., Zheludkevich M.L., et al. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part III—Inhibitors. Materials. 2022;15:8489. doi: 10.3390/ma15238489. - DOI - PMC - PubMed
-
- Vaghefinazari B., Wierzbicka E., Visser P., Posner R., Jordens G., Arrabal R., Matykina E., Mohedano M., Blawert C., Zheludkevich M.L., et al. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part I—Pre-Treatment and Conversion Coatings. Materials. 2022 in press. - PMC - PubMed
-
- Arrabal R., Mohedano M., Matykina E. Electrochemical Surface Treatments for Mg Alloys. In: Caballero F.G., editor. Encyclopedia of Materials: Metals and Allloys. Elsevier; Oxford, UK: 2022. pp. 87–112.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

