Chromate-Free Corrosion Protection Strategies for Magnesium Alloys-A Review: PART I-Pre-Treatment and Conversion Coating
- PMID: 36500170
- PMCID: PMC9736347
- DOI: 10.3390/ma15238676
Chromate-Free Corrosion Protection Strategies for Magnesium Alloys-A Review: PART I-Pre-Treatment and Conversion Coating
Abstract
Corrosion protection systems based on hexavalent chromium are traditionally perceived to be a panacea for many engineering metals including magnesium alloys. However, bans and strict application regulations attributed to environmental concerns and the carcinogenic nature of hexavalent chromium have driven a considerable amount of effort into developing safer and more environmentally friendly alternative techniques that provide the desired corrosion protection performance for magnesium and its alloys. Part I of this review series considers the various pre-treatment methods as the earliest step involved in the preparation of Mg surfaces for the purpose of further anti-corrosion treatments. The decisive effect of pre-treatment on the corrosion properties of both bare and coated magnesium is discussed. The second section of this review covers the fundamentals and performance of conventional and state-of-the-art conversion coating formulations including phosphate-based, rare-earth-based, vanadate, fluoride-based, and LDH. In addition, the advantages and challenges of each conversion coating formulation are discussed to accommodate the perspectives on their application and future development. Several auspicious corrosion protection performances have been reported as the outcome of extensive ongoing research dedicated to the development of conversion coatings, which can potentially replace hazardous chromium(VI)-based technologies in industries.
Keywords: conversion coating; corrosion; hexavalent chromium; magnesium; pre-treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




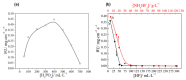






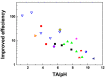






References
-
- Buchheit R.G., Hughes A.E. Chromate and Chromate-Free Conversion Coatings. ASM International; Almere, The Netherlands: 2003.
-
- ECHA Substances Restricted under REACH. 2022. [(accessed on 1 February 2022)]. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.239.176.
-
- Wierzbicka E., Vaghefinazari B., Mohedano M., Visser P., Posner R., Blawert C., Zheludkevich M., Lamaka S., Matykina E., Arrabal R. Chromate-Free Corrosion Protection Strategies for Magnesium Alloys—A Review: Part II—PEO and Anodizing. Materials. 2022;15:8515. doi: 10.3390/ma15238515. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

