Targeted Nanoparticles for the Binding of Injured Vascular Endothelium after Percutaneous Coronary Intervention
- PMID: 36500236
- PMCID: PMC9739478
- DOI: 10.3390/molecules27238144
Targeted Nanoparticles for the Binding of Injured Vascular Endothelium after Percutaneous Coronary Intervention
Abstract
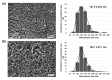
Percutaneous coronary intervention (PCI) is a common procedure for the management of coronary artery obstruction. However, it usually causes vascular wall injury leading to restenosis that limits the long-term success of the PCI endeavor. The ultimate objective of this study was to develop the targeting nanoparticles (NPs) that were destined for the injured subendothelium and attract endothelial progenitor cells (EPCs) to the damaged location for endothelium regeneration. Biodegradable poly(lactic-co-glycolic acid) (PLGA) NPs were conjugated with double targeting moieties, which are glycoprotein Ib alpha chain (GPIbα) and human single-chain antibody variable fragment (HuscFv) specific to the cluster of differentiation 34 (CD34). GPIb is a platelet receptor that interacts with the von Willebrand factor (vWF), highly deposited on the damaged subendothelial surface, while CD34 is a surface marker of EPCs. A candidate anti-CD34 HuscFv was successfully constructed using a phage display biopanning technique. The HuscFv could be purified and showed binding affinity to the CD34-positive cells. The GPIb-conjugated NPs (GPIb-NPs) could target vWF and prevent platelet adherence to vWF in vitro. Furthermore, the HuscFv-conjugated NPs (HuscFv-NPs) could capture CD34-positive cells. The bispecific NPs have high potential to locate at the damaged subendothelial surface and capture EPCs for accelerating the vessel repair.
Keywords: biodegradable nanoparticles; endothelium regeneration; percutaneous coronary intervention; single-chain antibody variable fragment; vascular injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- World Health Organization Cardiovascular Diseases (CVDs) [(accessed on 8 May 2022)]. Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-disea...
-
- Khan S.Q., Ludman P.F. Percutaneous coronary intervention. Medicine. 2022;50:437–444. doi: 10.1016/j.mpmed.2022.04.008. - DOI
MeSH terms
Substances
Grants and funding
- MRG6180180/Thailand Research Fund
- B05F640146/NSRF via the Program Management Unit for Human Resource & Institutional Development, Research and Innovation
- Research Network NANOTEC (RNN) program of the National Nanotechnology Center (NANOTEC), NSTDA, Ministry of Higher Education, Science, Research and Innovation (MHESI), Thailand
LinkOut - more resources
Full Text Sources
Miscellaneous

