A New MBH Adduct as an Efficient Ligand in the Synthesis of Metallodrugs: Characterization, Geometrical Optimization, XRD, Biological Activities, and Molecular Docking Studies
- PMID: 36500251
- PMCID: PMC9735827
- DOI: 10.3390/molecules27238150
A New MBH Adduct as an Efficient Ligand in the Synthesis of Metallodrugs: Characterization, Geometrical Optimization, XRD, Biological Activities, and Molecular Docking Studies
Abstract
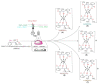
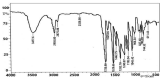
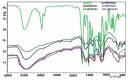
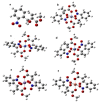
This article reports the synthesis, characterization, geometrical optimization, and biological studies of new MBH-based organometallic compounds of medicinal significance. The ligand (MNHA) was prepared via the Morita-Baylis-Hillman (MBH) synthetic route, from aromatic aldehyde containing multiple functional groups. Metal complexes were prepared in an alkaline medium and under other suitable reaction conditions. Spectral and elemental analyses were used to identify the structural and molecular formulas of each compound. Optimized geometry was determined through density functional theory (DFT) B3LYP and 6-311++ G (d,p) basis set for the MBH adduct, whereas structures of novel complexes were optimized with the semi-empirical PM6 method. Powder XRD analysis furnished the crystal class of complexes, with Co3+, Cr3+, and Mn2+ being cubic, while Ni2+ was hexagonal, and Cu2+ was orthorhombic. Moreover, the ligand, along with Ni2+ and Co3+ complexes, showed profound antibacterial action against S. aureus, E. coli, B. pumilis, and S. typhi. Additionally, all of the complexes were shown to persist in the positive antioxidant potential of the ligand. Contrarily, not a single metal complex conserved the antifungal potentials of the ligand.
Keywords: MBH adduct; XRD; biological studies; geometrical optimization; metal complexes.
Conflict of interest statement
There are no conflict of interest declared by all authors.
Figures






Similar articles
-
Synthesis, Characterization, Biological Activities and Ab-initio Study of Transition Metal Complexes of [Methyl 2-((4-chlorophenyl)(hydroxy)methyle) Acrylate].Acta Chim Slov. 2022 Jun 14;69(2):405-418. doi: 10.17344/acsi.2021.7305. Acta Chim Slov. 2022. PMID: 35861089
-
New Schiff base ligand and its novel Cr(III), Mn(II), Co(II), Ni(II), Cu(II), Zn(II) complexes: spectral investigation, biological applications, and semiconducting properties.Sci Rep. 2022 Oct 26;12(1):17942. doi: 10.1038/s41598-022-22713-z. Sci Rep. 2022. PMID: 36289280 Free PMC article.
-
Synthesis, structural characterization, and biological studies of ATBS-M complexes (M(II) = Cu, Co, Ni, and Mn): Access for promising antibiotics and anticancer agents.Arch Pharm (Weinheim). 2021 Apr;354(4):e2000241. doi: 10.1002/ardp.202000241. Epub 2020 Dec 18. Arch Pharm (Weinheim). 2021. PMID: 33336849
-
Synthesis, characterization and biological activities of Cu(II), Co(II), Mn(II), Fe(II), and UO2(VI) complexes with a new Schiff Base hydrazone: O-hydroxyacetophenone-7-chloro-4-quinoline hydrazone.Molecules. 2011 Oct 13;16(10):8629-45. doi: 10.3390/molecules16108629. Molecules. 2011. PMID: 21996717 Free PMC article.
-
Designing, characterization, biological, DFT, and molecular docking analysis for new FeAZD, NiAZD, and CuAZD complexes incorporating 1-(2-hydroxyphenylazo)- 2-naphthol (H2AZD).Comput Biol Chem. 2023 Aug;105:107908. doi: 10.1016/j.compbiolchem.2023.107908. Epub 2023 Jun 20. Comput Biol Chem. 2023. PMID: 37352589
References
-
- Sousa B.A., Dos Santos A.A. A Facile, Versatile, and Mild Morita-Baylis-Hillman-Type Reaction for the Modular One-Pot Synthesis of Highly Functionalized MBH Adducts. European J. Org. Chem. 2012:3431–3436. doi: 10.1002/ejoc.201200371. - DOI
-
- Du J.Y., Ma Y.H., Meng F.X., Zhang R.R., Wang R.N., Shi H.L., Wang Q., Fan Y.X., Huang H.L., Cui J.C., et al. Lewis Base-Catalyzed [4 + 3] Annulation of Ortho-Quinone Methides and MBH Carbonates: Synthesis of Functionalized Benzo[ b]Oxepines Bearing Oxindole Scaffolds. Org. Lett. 2019;21:465–468. doi: 10.1021/acs.orglett.8b03709. - DOI - PubMed
-
- Kaye P.T. Applications of the Morita–Baylis–Hillman Reaction in the Synthesis of Heterocyclic Systems. 1st ed. Volume 127. Elsevier Inc.; Amsterdam, The Netherlands: 2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

