Quantification of Tafenoquine and 5,6-Orthoquinone Tafenoquine by UHPLC-MS/MS in Blood, Plasma, and Urine, and Application to a Pharmacokinetic Study
- PMID: 36500278
- PMCID: PMC9737280
- DOI: 10.3390/molecules27238186
Quantification of Tafenoquine and 5,6-Orthoquinone Tafenoquine by UHPLC-MS/MS in Blood, Plasma, and Urine, and Application to a Pharmacokinetic Study
Abstract
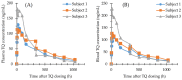
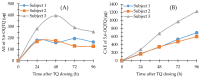
Analytical methods for the quantification of the new 8-aminoquinoline antimalarial tafenoquine (TQ) in human blood, plasma and urine, and the 5,6-orthoquinone tafenoquine metabolite (5,6-OQTQ) in human plasma and urine have been validated. The procedure involved acetonitrile extraction of samples followed by ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS). Chromatography was performed using a Waters Atlantis T3 column with a gradient of 0.1% formic acid and acetonitrile at a flow rate of 0.5 mL per minute for blood and plasma. Urine analysis was the same but with methanol containing 0.1% formic acid replacing acetonitrile mobile phase. The calibration range for TQ and 5,6-OQTQ in plasma was 1 to 1200 ng/mL, and in urine was 10 to 1000 ng/mL. Blood calibration range for TQ was 1 to 1200 ng/mL. Blood could not be validated for 5,6-OQTQ due to significant signal suppression. The inter-assay precision (coefficient of variation %) was 9.9% for TQ at 1 ng/mL in blood (n = 14) and 8.2% for TQ and 7.1% for 5,6-OQTQ at 1 ng/mL in plasma (n = 14). For urine, the inter-assay precision was 8.2% for TQ and 6.4% for 5,6-OQTQ at 10 ng/mL (n = 14). TQ and 5,6-OQTQ are stable in blood, plasma and urine for at least three months at both -80 °C and -20 °C. Once validated, the analytical methods were applied to samples collected from healthy volunteers who were experimentally infected with Plasmodium falciparum to evaluate the blood stage antimalarial activity of TQ and to determine the therapeutic dose estimates for TQ, the full details of which will be published elsewhere. In this study, the measurement of TQ and 5,6-OQTQ concentrations in samples from one of the four cohorts of participants is reported. Interestingly, TQ urine concentrations were proportional to parasite recrudescence times post dosing To our knowledge, this is the first description of a fully validated method for the measurement of TQ and 5,6-OQTQ quantification in urine.
Keywords: 5,6-orthoquinone tafenoquine; analytical method; blood; malaria; plasma; tafenoquine; urine.
Conflict of interest statement
The authors declare no conflict of interest. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Australian Defence Force, Joint Health Command or any extant Australian Defence Force policy.
Figures



References
-
- Fasinu P.S., Nanayakkara N.P.D., Wang Y.H., Chaurasiya N.D., Herath H.M.B., McChesney J.D., Avula B., Khan I., Tekwani B.L., Walker L.A. Formation primaquine-5,6-orthoquinone, the putative active and toxic metabolite of primaquine via direct oxidation in human erythrocytes. Malar. J. 2019;18:30. doi: 10.1186/s12936-019-2658-5. - DOI - PMC - PubMed
-
- Alving A.S., Carson P.E., Flanagan C.L., Ickes C.E. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science. 1956;124:484–485. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

