N-Arylation of 3-Formylquinolin-2(1 H)-ones Using Copper(II)-Catalyzed Chan-Lam Coupling
- PMID: 36500438
- PMCID: PMC9735505
- DOI: 10.3390/molecules27238345
N-Arylation of 3-Formylquinolin-2(1 H)-ones Using Copper(II)-Catalyzed Chan-Lam Coupling
Abstract
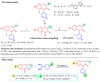
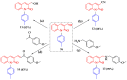
3-formyl-2-quinolones have attracted the scientific community's attention because they are used as versatile building blocks in the synthesis of more complex compounds showing different and attractive biological activities. Using copper-catalyzed Chan-Lam coupling, we synthesized 32 new N-aryl-3-formyl-2-quinolone derivatives at 80 °C, in air and using inexpensive phenylboronic acids as arylating agents. 3-formyl-2-quinolones and substituted 3-formyl-2-quinolones can act as substrates, and among the products, the p-methyl derivative 9a was used as a substrate to obtain different derivatives such as alcohol, amine, nitrile, and chalcone.
Keywords: 3-formylquinolones; Chan–Lam coupling; N-arylation; copper(II).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
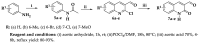
Similar articles
-
Synthesis of N-Arylcytisine Derivatives Using the Copper-Catalyzed Chan-Lam Coupling.J Nat Prod. 2021 Jul 23;84(7):1985-1992. doi: 10.1021/acs.jnatprod.1c00275. Epub 2021 Jul 2. J Nat Prod. 2021. PMID: 34213336
-
Cu-Catalyzed Chan-Evans-Lam Coupling Reactions of 2-Nitroimidazole with Aryl Boronic Acids: An Effort toward New Bioactive Agents against S. pneumoniae.Chem Biodivers. 2022 Aug;19(8):e202200327. doi: 10.1002/cbdv.202200327. Epub 2022 Aug 3. Chem Biodivers. 2022. PMID: 35819995 Free PMC article.
-
N,N'-Dimethylurea as an efficient ligand for the synthesis of pharma-relevant motifs through Chan-Lam cross-coupling strategy.Org Biomol Chem. 2023 Apr 12;21(15):3143-3155. doi: 10.1039/d3ob00176h. Org Biomol Chem. 2023. PMID: 36987866
-
Recent Developments in Arylation of N-Nucleophiles via Chan-Lam Reaction: Updates from 2012 Onwards.Curr Org Synth. 2022;19(1):16-30. doi: 10.2174/1570179417666210105120706. Curr Org Synth. 2022. PMID: 33402088 Review.
-
Synthetic applications and methodology development of Chan-Lam coupling: a review.Mol Divers. 2019 Feb;23(1):215-259. doi: 10.1007/s11030-018-9870-z. Epub 2018 Aug 30. Mol Divers. 2019. PMID: 30159807 Review.
Cited by
-
Natural protection against oxidative stress in human skin melanocytes.Commun Biol. 2025 Aug 26;8(1):1283. doi: 10.1038/s42003-025-08725-1. Commun Biol. 2025. PMID: 40858814 Free PMC article. Review.
References
-
- Shobeiri N., Rashedi M., Mosaffa F., Zarghi A., Ghandadi M., Ghasemi A., Ghodsi R. Synthesis and Biological Evaluation of Quinoline Analogues of Flavones as Potential Anticancer Agents and Tubulin Polymerization Inhibitors. Eur. J. Med. Chem. 2016;114:14–23. doi: 10.1016/j.ejmech.2016.02.069. - DOI - PubMed
-
- Kappenberg Y.G., Ketzer A., Stefanello F.S., Salbego P.R.S., Acunha T.V., Abbadi B.L., Bizarro C.V., Basso L.A., Machado P., Martins M.A.P., et al. Synthesis and Photophysical, Thermal and Antimycobacterial Properties of Novel 6-Amino-2-Alkyl(Aryl/Heteroaryl)-4-(Trifluoromethyl) Quinolines. New J. Chem. 2019;43:12375–12384. doi: 10.1039/C9NJ01681C. - DOI
-
- Bonacorso H.G., Rodrigues M.B., Iglesias B.A., da Silveira C.H., Feitosa S.C., Rosa W.C., Martins M.A.P., Frizzo C.P., Zanatta N. New 2-(Aryl/Heteroaryl)-6-(Morpholin-4-Yl/Pyrrolidin-1-Yl)-(4-Trifluoromethyl)Quinolines: Synthesis via Buchwald–Hartwig Amination, Photophysics, and Biomolecular Binding Properties. New J. Chem. 2018;42:10024–10035. doi: 10.1039/C8NJ01120F. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

