Fluorescent Organic Small Molecule Probes for Bioimaging and Detection Applications
- PMID: 36500513
- PMCID: PMC9737913
- DOI: 10.3390/molecules27238421
Fluorescent Organic Small Molecule Probes for Bioimaging and Detection Applications
Abstract
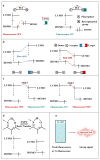
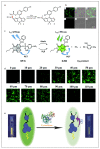
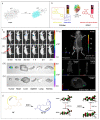
The activity levels of key substances (metal ions, reactive oxygen species, reactive nitrogen, biological small molecules, etc.) in organisms are closely related to intracellular redox reactions, disease occurrence and treatment, as well as drug absorption and distribution. Fluorescence imaging technology provides a visual tool for medicine, showing great potential in the fields of molecular biology, cellular immunology and oncology. In recent years, organic fluorescent probes have attracted much attention in the bioanalytical field. Among various organic fluorescent probes, fluorescent organic small molecule probes (FOSMPs) have become a research hotspot due to their excellent physicochemical properties, such as good photostability, high spatial and temporal resolution, as well as excellent biocompatibility. FOSMPs have proved to be suitable for in vivo bioimaging and detection. On the basis of the introduction of several primary fluorescence mechanisms, the latest progress of FOSMPs in the applications of bioimaging and detection is comprehensively reviewed. Following this, the preparation and application of fluorescent organic nanoparticles (FONPs) that are designed with FOSMPs as fluorophores are overviewed. Additionally, the prospects of FOSMPs in bioimaging and detection are discussed.
Keywords: bioimaging; detection; fluorescent organic nanoparticles; fluorescent organic small molecules; recognition mechanisms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Fukuto J.M., Carrington S.J., Tantillo D.J., Harrison J.G., Ignarro L.J., Freeman B.A., Chen A., Wink D.A. Small Molecule Signaling Agents: The Integrated Chemistry and Biochemistry of Nitrogen Oxides, Oxides of Carbon, Dioxygen, Hydrogen Sulfide, and Their Derived Species. Chem. Res. Toxicol. 2012;25:769–793. doi: 10.1021/tx2005234. - DOI - PMC - PubMed
-
- Griendling K.K., Touyz R.M., Zweier J.L., Dikalov S., Chilian W., Chen Y., Harrison D.G., Bhatnagar A., Amer H.A.C.B. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement From the American Heart Association. Circ. Res. 2016;119:E39–E75. doi: 10.1161/RES.0000000000000110. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 52101287/National Natural Science Foundation of China
- U1806219/National Natural Science Foundation of China
- 2020GXRC019/Special Funding in the Project of the Taishan Scholar Construction Engineering and the program of Jinan Science and Technology Bureau
- 2020-370104-34-03-043952-01-11/New material demonstration platform construction project from Ministry of Industry and Infor-mation Technology
LinkOut - more resources
Full Text Sources

