Azumamides A-E: Isolation, Synthesis, Biological Activity, and Structure-Activity Relationship
- PMID: 36500529
- PMCID: PMC9737774
- DOI: 10.3390/molecules27238438
Azumamides A-E: Isolation, Synthesis, Biological Activity, and Structure-Activity Relationship
Abstract
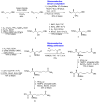
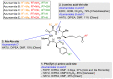
Cyclic peptides are one of the important chemical groups in the HDAC inhibitor family. Following the success of romidepsin in the clinic, naturally occurring cyclic peptides with a hydrophilic moiety have been intensively studied to test their function as HDAC inhibitors. Azumamides A-E, isolated from Mycale izuensis, are one of the powerful HDAC inhibitor classes. Structurally, azumamides A-E consist of three D-α-amino acids and unnatural β-amino acids such as 3-amino-2-methyl-5-nonenedioic acid-9-amide (Amnna) and 3-amino-2-methyl-5-nonenoic-1,9-diacid (Amnda). Moreover, azumamides have a retro-arrangement peptide backbone, unlike other naturally occurring cyclopeptide HDAC inhibitors, owing to the D-configuration of all residues. This review summarizes the currently available synthetic methods of azumamides A-E focusing on the synthesis of β-amino acids and macrocyclization. In addition, we overview the structure-activity relationship of azumamides A-E based on reported analogs. Collectively, this review highlights the potentiality of azumamides A-E as an HDAC inhibitor and provides further developmental insight into naturally occurring cyclic peptides in HDAC inhibition.
Keywords: HDAC inhibitor; asymmetric synthesis; azumamide; macrocyclization; naturally occurring cyclic peptide; β-amino acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

