Copper-Catalyzed Difluoroalkylation Reaction
- PMID: 36500553
- PMCID: PMC9740754
- DOI: 10.3390/molecules27238461
Copper-Catalyzed Difluoroalkylation Reaction
Abstract
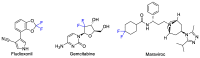
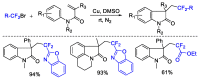
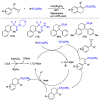
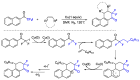
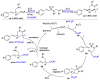
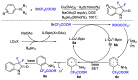
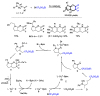
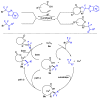
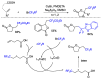
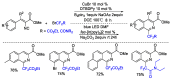
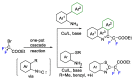
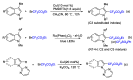
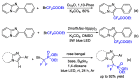
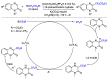
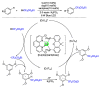
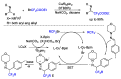
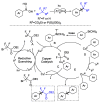
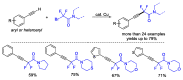
This review describes recent advances in copper-catalyzed difluoroalkylation reactions. The RCF2 radical is generally proposed in the mechanism of these reactions. At present, various types of copper-catalyzed difluoroalkylation reactions have been realized. According to their characteristics, we classify these difluoroalkylation reactions into three types.
Keywords: copper; coupling reaction; cyclization; difluoroalkylation; multicomponent; radical.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


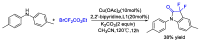




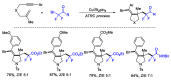


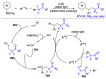

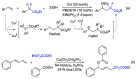


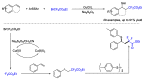

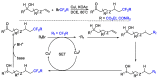
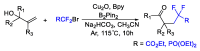
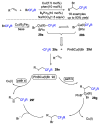
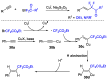
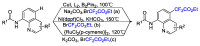



References
-
- Lasing T., Phumee A., Siriyasatien P., Chitchak K., Vanalabhpatana P., Mak K.K., Hee Ng C., Vilaivan T., Khotavivattana T. Synthesis and antileishmanial activity of fluorinated rhodacyanine analogues: The ‘fluorine-walk’ analysis. Bioorg. Med. Chem. 2020;28:115187. doi: 10.1016/j.bmc.2019.115187. - DOI - PubMed
-
- Cech R., Zaller J.G., Lyssimachou A., Clausing P., Hertoge K., Linhart C. Pesticide drift mitigation measures appear to reduce contamination of non-agricultural areas, but hazards to humans and the environment remain. Sci. Total Environ. 2023;854:158814. doi: 10.1016/j.scitotenv.2022.158814. - DOI - PubMed
-
- Xiao X., Cai H., Huang Q., Wang B., Wang X., Luo Q., Li Y., Zhang H., Gong Q., Ma X., et al. Polymeric dual-modal imaging nanoprobe with two-photon aggregation-induced emission for fluorescence imaging and gadolinium-chelation for magnetic resonance imaging. Bioact. Mater. 2023;19:538–549. doi: 10.1016/j.bioactmat.2022.04.026. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

