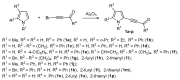
Substituent-Dependent Divergent Synthesis of 2-(3-Amino-2,4-dicyanophenyl)pyrroles, Pyrrolyldienols and 3-Amino-1-acylethylidene-2-cyanopyrrolizines via Reaction of Acylethynylpyrroles with Malononitrile
- PMID: 36500621
- PMCID: PMC9737003
- DOI: 10.3390/molecules27238528
Substituent-Dependent Divergent Synthesis of 2-(3-Amino-2,4-dicyanophenyl)pyrroles, Pyrrolyldienols and 3-Amino-1-acylethylidene-2-cyanopyrrolizines via Reaction of Acylethynylpyrroles with Malononitrile
Abstract
An efficient method for the synthesis of pharmaceutically and high-tech prospective 2-(3-amino-2,4-dicyanophenyl)pyrroles (in up to 88% yield) via the reaction of easily available substituted acylethynylpyrroles with malononitrile has been developed. The reaction proceeds in the KOH/MeCN system at 0 °C for 2 h. In the case of 2-acylethynylpyrroles without substituents in the pyrrole ring, the reaction changes direction: instead of the target 2-(3-amino-2,4-dicyanophenyl)pyrroles, the unexpected formation of pyrrolyldienols and products of their intramolecular cyclization, 3-amino-1-acylethylidene-2-cyanopyrrolizines, is observed.
Keywords: 2-(3-amino-2,4-dicyanophenyl)pyrroles; 3-amino-1-acylethylidene-2-cyanopyrrolizines; acylethynylpyrroles; malononitrile; pyrrolyldienols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





 ) and HMBC (
) and HMBC ( ) correlations for pyrolizine 6c.
) correlations for pyrolizine 6c.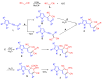

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

