Polysaccharide-Based Composite Hydrogels as Sustainable Materials for Removal of Pollutants from Wastewater
- PMID: 36500664
- PMCID: PMC9736407
- DOI: 10.3390/molecules27238574
Polysaccharide-Based Composite Hydrogels as Sustainable Materials for Removal of Pollutants from Wastewater
Abstract
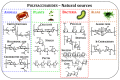
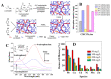
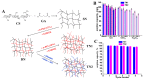
Nowadays, pollution has become the main bottleneck towards sustainable technological development due to its detrimental implications in human and ecosystem health. Removal of pollutants from the surrounding environment is a hot research area worldwide; diverse technologies and materials are being continuously developed. To this end, bio-based composite hydrogels as sorbents have received extensive attention in recent years because of advantages such as high adsorptive capacity, controllable mechanical properties, cost effectiveness, and potential for upscaling in continuous flow installations. In this review, we aim to provide an up-to-date analysis of the literature on recent accomplishments in the design of polysaccharide-based composite hydrogels for removal of heavy metal ions, dyes, and oxyanions from wastewater. The correlation between the constituent polysaccharides (chitosan, cellulose, alginate, starch, pectin, pullulan, xanthan, salecan, etc.), engineered composition (presence of other organic and/or inorganic components), and sorption conditions on the removal performance of addressed pollutants will be carefully scrutinized. Particular attention will be paid to the sustainability aspects in the selected studies, particularly to composite selectivity and reusability, as well as to their use in fixed-bed columns and real wastewater applications.
Keywords: adsorption; hydrogels; polysaccharides; sustainable development; wastewater treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




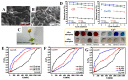
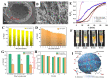

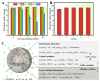

References
-
- United Nations General Assembly . Transforming Our World: The 2020 Agenda for Sustainable Development. United Nations General Assembly; New York, NY, USA: 2015.
-
- Akhtar N., Ishak M.I.S., Bhawani S.A., Umar K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water. 2021;13:2660. doi: 10.3390/w13192660. - DOI
-
- Saravan A., Senthil Kumar P., Jeevanantham S., Karishma S., Tajsabreen B., Yaashikaa P.R., Reshma B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere. 2021;280:130595. doi: 10.1016/j.chemosphere.2021.130595. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

