Dietary Emulsifiers Exacerbate Food Allergy and Colonic Type 2 Immune Response through Microbiota Modulation
- PMID: 36501013
- PMCID: PMC9738911
- DOI: 10.3390/nu14234983
Dietary Emulsifiers Exacerbate Food Allergy and Colonic Type 2 Immune Response through Microbiota Modulation
Abstract
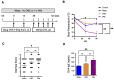
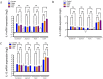
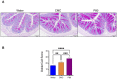
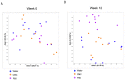
The significant increase in food allergy incidence is correlated with dietary changes in modernized countries. Here, we investigated the impact of dietary emulsifiers on food allergy by employing an experimental murine model. Mice were exposed to drinking water containing 1.0% carboxymethylcellulose (CMC) or Polysorbate-80 (P80) for 12 weeks, a treatment that was previously demonstrated to induce significant alterations in microbiota composition and function leading to chronic intestinal inflammation and metabolic abnormalities. Subsequently, the ovalbumin food allergy model was applied and characterized. As a result, we observed that dietary emulsifiers, especially P80, significantly exacerbated food allergy symptoms, with increased OVA-specific IgE induction and accelerated type 2 cytokine expressions, such as IL-4, IL-5, and IL-13, in the colon. Administration of an antibiotic regimen completely reversed the emulsifier-induced exacerbated susceptibility to food allergy, suggesting a critical role played by the intestinal microbiota in food allergy and type 2 immune responses.
Keywords: dietary emulsifiers; food allergy; microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

