p-Hydroxybenzyl Alcohol Antagonized the ROS-Dependent JNK/Jun/Caspase-3 Pathway to Produce Neuroprotection in a Cellular Model of Parkinson's Disease
- PMID: 36501032
- PMCID: PMC9741417
- DOI: 10.3390/nu14235002
p-Hydroxybenzyl Alcohol Antagonized the ROS-Dependent JNK/Jun/Caspase-3 Pathway to Produce Neuroprotection in a Cellular Model of Parkinson's Disease
Abstract
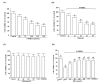
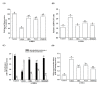
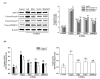
Parkinson's disease (PD) is a progressive disorder that affects brain nerve cells responsible for body motion and remains incurable. p-Hydroxybenzyl alcohol (HBA) is the primary phenolic compound in Gastrodiae Rhizoma, known for its therapeutic benefits against neurodegeneration. However, the protective effect of HBA against Parkinson's disease (PD) remains unclear. The objective of this study was to evaluate the neuroprotective effects of HBA in vitro 6-hydroxydopamine (6-OHDA)-induced PD model in SH-SY5Y cells. SH-SY5Y cells were pretreated with various concentrations of HBA for 1 h and incubated with 100 μmol/L 6-OHDA for 24 h to induce cellular lesions. 2,5-Diphenyl-2H-tetrazolium bromide was used to detect cellular viability. 2',7'-dichlorofluorescin oxidation detects reactive oxygen species (ROS). The enzyme-linked immunosorbent assay was used to determine the activities of superoxide dismutase, catalase, and glutathione peroxidase. The cellular mitochondrial function was identified through the collapse of the mitochondrial membrane potential, the release of cytochrome c, and the synthesis of mitochondrial ATP. Expression of pro-and anti-apoptotic factors was measured by Western blot. HBA enhanced cell viability, blocked ROS overproduction, and reduced antioxidant activities induced by 6-OHDA. HBA also reduced mitochondrial dysfunction and cell death caused by 6-OHDA. Moreover, HBA reversed the 6-OHDA-mediated activation of c-Jun N-terminal kinase, the downregulation of the Bcl-2/Bax ratio, the Apaf-1 upregulation and the induction of caspase-9, caspase-3, and PARP cleavage. This study shows that the protective effects of HBA against 6-OHDA-induced cell injury provide the potential preventive effects of HBA, making it a promising preventive agent for PD.
Keywords: 6-OHDA; Parkinson’s disease; SH-SY5Y cells; neurodegeneration; p-hydroxybenzyl alcohol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

