A Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Effects of Multi-Strain Synbiotic in Patients with Functional Diarrhea and High Fecal Calprotectin Levels: A Pilot Study
- PMID: 36501047
- PMCID: PMC9735760
- DOI: 10.3390/nu14235017
A Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Effects of Multi-Strain Synbiotic in Patients with Functional Diarrhea and High Fecal Calprotectin Levels: A Pilot Study
Abstract
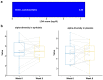
Synbiotics, including probiotics and prebiotics, are useful for patients with functional bowel disorders. However, which synbiotics are beneficial for patients with which diseases, especially those with functional diarrhea (FDr) with high fecal calprotectin levels, is currently unknown. FDr is an extension of irritable bowel syndrome with diarrhea (IBS-D). Although fewer studies have been conducted on FDr compared to IBS-D, its importance is increasing as its prevalence increases. The aim of this study was to evaluate the effects of a synbiotic containing a mixture of Lactobacillus and Bifidobacterium and its substrate, fructooligosaccharide, on bowel symptoms, fecal calprotectin levels, fecal microbiota, and safety in FDr patients with high fecal calprotectin levels. Forty patients were randomly assigned to either a synbiotic group or a placebo group. A total of 20 subjects in the synbiotic group and 19 subjects in the placebo group completed the study (8 weeks). Changes in FDr symptoms, fecal calprotectin levels, and gut microbiota were assessed during the intervention period. At 4 and 8 weeks, the number of bowel movements tended to increase in the synbiotic group, with a significant increase in the number of formed stools rather than loose stools (p < 0.05). Bowel movement satisfaction was significantly increased in the synbiotic group, but not in the placebo group. Intestinal flora analysis revealed that Lactobacillales at the order level was increased only in the synbiotic group at the end of the intervention. In contrast, at week 8 of the intervention, log-transformed fecal calprotectin levels were significantly decreased in the synbiotic group, although the change was not significantly different from that of the placebo group. These findings suggest that the intake of a multi-strain-containing synbiotic for 8 weeks could improve gut symptoms and the intestinal microenvironment of FDr patients with high fecal calprotectin levels.
Keywords: Bifidobacterium; Lactobacillus; fecal calprotectin; fructooligosaccharides; functional diarrhea; multi-strain synbiotic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sperber A.D., Bangdiwala S.I., Drossman D.A., Ghoshal U.C., Simren M., Tack J., Whitehead W.E., Dumitrascu D.L., Fang X., Fukudo S., et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology. 2021;160:99–114.e3. doi: 10.1053/j.gastro.2020.04.014. - DOI - PubMed
-
- Carroll I.M., Ringel-Kulka T., Keku T.O., Chang Y.H., Packey C.D., Sartor R.B., Ringel Y. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G799–G807. doi: 10.1152/ajpgi.00154.2011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

