Puerariae lobatae Radix Alleviates Pre-Eclampsia by Remodeling Gut Microbiota and Protecting the Gut and Placental Barriers
- PMID: 36501055
- PMCID: PMC9738998
- DOI: 10.3390/nu14235025
Puerariae lobatae Radix Alleviates Pre-Eclampsia by Remodeling Gut Microbiota and Protecting the Gut and Placental Barriers
Abstract
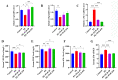
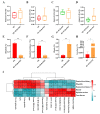
Pre-eclampsia (PE) is a serious pregnancy complication, and gut dysbiosis is an important cause of it. Puerariae lobatae Radix (PLR) is a medicine and food homologous species; however, its effect on PE is unclear. This study aimed to investigate the efficacy of PLR in alleviating PE and its mechanisms. We used an NG-nitro-L-arginine methyl ester (L-NAME)-induced PE mouse model to examine the efficacy of preventive and therapeutic PLR supplementation. The results showed that both PLR interventions alleviated hypertension and proteinuria, increased fetal and placental weights, and elevated the levels of VEGF and PlGF. Moreover, PLR protected the placenta from oxidative stress via activating the Nrf2/HO-1/NQO1 pathway and mitigated placental damage by increasing intestinal barrier markers (ZO-1, Occludin, and Claudin-1) expression and reducing lipopolysaccharide leakage. Notably, preventive PLR administration corrected gut dysbiosis in PE mice, as evidenced by the increased abundance and positive interactions of beneficial bacteria including Bifidobacterium, Blautia, and Turicibacter. Fecal microbiota transplantation confirmed that the gut microbiota partially mediated the beneficial effects of PLR on PE. Our findings revealed that modulating the gut microbiota is an effective strategy for the treatment of PE and highlighted that PLR might be used as an intestinal nutrient supplement in PE patients.
Keywords: Puerariae lobatae Radix; fecal microflora transplantation; gut microbiota; gut-placenta axis; pre-eclampsia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Brown M.A., Magee L.A., Kenny L.C., Karumanchi S.A., McCarthy F., Saito S., Hall D.R., Warren C.E., Adoyi G., Ishaku S. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

