Ramulus Mori (Sangzhi) Alkaloids Ameliorate Obesity-Linked Adipose Tissue Metabolism and Inflammation in Mice
- PMID: 36501080
- PMCID: PMC9739644
- DOI: 10.3390/nu14235050
Ramulus Mori (Sangzhi) Alkaloids Ameliorate Obesity-Linked Adipose Tissue Metabolism and Inflammation in Mice
Abstract
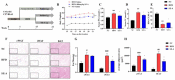
Obesity has become a global epidemic disease as it is closely associated with a chronic low-grade inflammatory state that results in metabolic dysfunction. Ramulus Mori (Sangzhi) alkaloids (SZ-A) derived from Morus alba L. were licensed to treat type 2 diabetes (T2DM) in 2020. In this study, we explored the effect of SZ-A on adipose tissue metabolism and inflammation using an obesity model induced by a high-fat diet (HFD). C57BL/6J mice were fed high fat for 14 weeks and followed by SZ-A 400 mg/kg treatment via gavage for another six weeks, during which they were still given the high-fat diet. The results showed that SZ-A notably reduced body weight and serum levels of lipid metabolism-related factors, such as triglycerides (TG) and total cholesterol (TC); and inflammation-related factors, namely tumor necrosis factor alpha (TNFα), interleukin 6 (IL6), fibrinogen activator inhibitor-1 (PAI-1), angiopoietin-2 (Ang-2), and leptin (LEP), in the HFD-induced mice. SZ-A increased the protein and mRNA expression of lipid metabolism-related factors, including phosphorylated acetyl coenzyme A carboxylase (p-ACC), phosphorylated hormone-sensitive triglyceride lipase (p-HSL), adipose triglyceride lipase (ATGL), and peroxisome proliferator-activated receptor-alpha (PPARα), in adipose tissue. Immunohistochemistry results demonstrated that SZ-A significantly reduced the infiltration of pro-inflammatory M1-type macrophages in epididymal fat. The data also suggested that SZ-A down-regulates the transcriptional levels of inflammatory factors Il6, Tnfα, monocyte chemoattractant protein-1 (Mcp1), and F4/80, and up-regulates interleukin 4 (Il4), interleukin 10 (Il10), and interleukin 13 (Il13) in adipose tissue. Overall, the results indicate that SZ-A exhibits potential in regulating lipid metabolism and ameliorating obesity-linked adipose inflammation.
Keywords: Ramulus Mori alkaloids; adipose inflammation; adipose tissue; lipid metabolism; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

