Human Milk Oligosaccharides as Potential Antibiofilm Agents: A Review
- PMID: 36501142
- PMCID: PMC9737902
- DOI: 10.3390/nu14235112
Human Milk Oligosaccharides as Potential Antibiofilm Agents: A Review
Abstract
Surface-associated bacterial communities called biofilms are ubiquitous in nature. Biofilms are detrimental in medical settings due to their high tolerance to antibiotics and may alter the final pathophysiological outcome of many healthcare-related infections. Several innovative prophylactic and therapeutic strategies targeting specific mechanisms and/or pathways have been discovered and exploited in the clinic. One such emerging and original approach to dealing with biofilms is the use of human milk oligosaccharides (HMOs), which are the third most abundant solid component in human milk after lactose and lipids. HMOs are safe to consume (GRAS status) and act as prebiotics by inducing the growth and colonization of gut microbiota, in addition to strengthening the intestinal epithelial barrier, thereby protecting from pathogens. Moreover, HMOs can disrupt biofilm formation and inhibit the growth of specific microbes. In the present review, we summarize the potential of HMOs as antibacterial and antibiofilm agents and, hence, propose further investigations on using HMOs for new-age therapeutic interventions.
Keywords: antimicrobial resistance; biofilm; gut microbiota; human milk oligosaccharides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

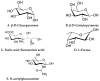
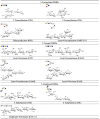

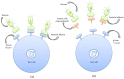

References
-
- Bhowmik A., Malhotra A., Jana S., Chauhan A. Biofilm Formation and Pathogenesis. Spinger; Berlin/Heidelberg, Germany: 2021. pp. 3–37.
-
- Singh B.P., Ghosh S., Chauhan A. Development, dynamics and control of antimicrobial-resistant bacterial biofilms: A review. Environ. Chem. Lett. 2021;19:1983–1993. doi: 10.1007/s10311-020-01169-5. - DOI
-
- da Silva R.A.G., Afonina I., Kline K.A. Eradicating biofilm infections: An update on current and prospective approaches. Curr. Opin. Microbiol. 2021;63:117–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

