DNA Barcoding and Morphometry Reveal Further Cryptic Bio-Diversity within the Pin Nematode Genus Paratylenchus (Nematoda: Tylenchulidae)
- PMID: 36501423
- PMCID: PMC9735703
- DOI: 10.3390/plants11233385
DNA Barcoding and Morphometry Reveal Further Cryptic Bio-Diversity within the Pin Nematode Genus Paratylenchus (Nematoda: Tylenchulidae)
Abstract
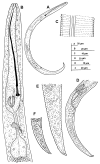
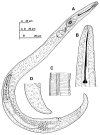
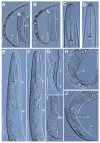
Paratylenchus species are obligate ectoparasitic nematodes on cultivated and wild herbaceous and woody plants occupying numerous soil categories. Several species may cause damage to several crops (viz. P. dianthus, P. enigmaticus, P. microdorus, P. hamatus and P. epacris on carnation, lettuce, rose and walnut, respectively). This investigation proves and emphasizes the relevance of applying integrative taxonomy for the accurate detection of Paratylenchus species in mountainous wild environments in the Malaga province, Southern Spain. This research analyzed 45 soil samples of maritimus pine and one of green heather in southern Spain and identified fourteen Paratylenchus species, two of them are described herein as new species (P. paraaonli sp. nov., P. plesiostraeleni sp. nov.), six of them were first reports for Spain (P. canchicus, P. nainianus, P. neonanus, P. salubris, Paratylenchus sp. 2 SAS, and P. wuae), and six species (P. caravaquenus, P. microdorus, P. nanus, P. neoamblycephalus, P. sheri, and P. variabilis) have been already reported in Spain. Accordingly, these data increase the biodiversity of pin nematodes in Spain comprising a total of 47 species (33.1% out of 142 total species of this genus). Phylogenetic analyses based on ribosomal and mitochondrial markers (D2-D3, ITS, and partial COI) resulted in a consistent position for the newly described Paratylenchus species in this study (P. plesiostraeleni sp. nov., P. paraaonli sp. nov.). Paratylenchus plesiostraeleni sp. nov. grouped in a separated subclade as unequivocal species from the P. straeleni-complex species (including P. straeleni and P. parastraeleni), and P. paraaonli sp. nov. clustered with P. vitecus, but clearly separate from this species. This study indicates that Paratylenchus species diversity in natural environments may be higher than expected, and this study may help in accurate identifications.
Keywords: D2-D3 expansion domains of the large ribosomal subunit (28S); cryptic species; cytochrome c oxidase c subunit 1 (COI); internal transcribed spacer (ITS); new species.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures













References
-
- Micoletzky H. Die freilebenden Erd-Nematoden. Arch. Für Nat. 1922;87:1–650.
-
- Ghaderi R., Geraert E., Karegar A. The Tylenchulidae of the World, Identification of the Family Tylenchulidae (Nematoda: Tylenchida) Academia Press; Ghent, Belgium: 2016.
-
- Clavero-Camacho I., Palomares-Rius J.E., Cantalapiedra-Navarrete C., Leon-Ropero G., Martin-Barbarroja J., Archidona-Yuste A., Castillo P. Integrative taxonomy reveals hidden cryptic diversity within pin nematodes of the genus Paratylenchus (Nematoda: Tylenchulidae) Plants. 2021;10:1454. doi: 10.3390/plants10071454. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

