Negative regulation of interferon regulatory factor 6 (IRF6) in interferon and NF-κB signalling pathways of common carp (Cyprinus carpio L.)
- PMID: 36503433
- PMCID: PMC9743528
- DOI: 10.1186/s12917-022-03538-4
Negative regulation of interferon regulatory factor 6 (IRF6) in interferon and NF-κB signalling pathways of common carp (Cyprinus carpio L.)
Abstract
Background: Interferon (IFN) regulatory factors (IRFs) is a kind of transcription factors, which play an important role in regulating the expression of type I IFN and related genes. In mammals, IRF6 is not relevant with IFN expression, while zebrafish IRF6 was reported to be a positive regulator of IFN expression and could be phosphorylated by both MyD88 and TBK1. However, the role of IRF6 in the immune response and IFN transcription of common carp is unknown.
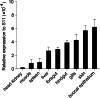
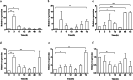
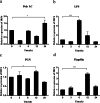
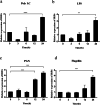
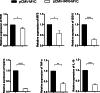
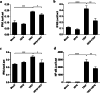
Results: In the present study, the cDNA of IRF6 gene (CcIRF6) was cloned from common carp using RACE technique, with a total length of 1905 bp, encoding 471 amino acid residues, which possesses two functional domains of DBD and IAD. Similarity analysis showed that CcIRF6 had more than 50% similarity with IRFs of other vertebrates, and had the highest similarity with grass carp and zebrafish, among which the DBD domain was much more conserved. The phylogenetic analysis showed that CcIRF6 is in the branch of Osteichthyes and has the closest relationship with grass carp. In healthy common carp, the CcIRF6 was expressed in all the examined tissues, with the highest level in the oral epithelium, and the lowest level in the head kidney. After intraperitoneal injection of poly(I:C) or Aeromonas hydrophila, the expression of CcIRF6 increased in spleen, head kidney, foregut and hindgut of common carp. Moreover, poly(I:C), LPS, PGN and flagellin induced the expression of CcIRF6 in peripheral leukocytes and head kidney leukocytes of common carp in vitro. In EPC cells, CcIRF6 inhibited the expression of some IFN-related genes and pro-inflammatory cytokines, and dual luciferase reporter assay showed that CcIRF6 reduced the activity of IFN and NF-κB reporter genes.
Conclusions: The present study suggests that CcIRF6 is involved in the antiviral and antibacterial immune response of common carp, and negatively regulate the expression of IFN and NF-κB signalling pathways, which provides a theoretical basis for the study and prevention of fish disease pathogenesis.
Keywords: Aeromonas hydrophila; Common carp (Cyprinus carpio L.); Interferon; Interferon regulatory factor 6 (IRF6); NF-κB; Poly(I:C).
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- 32002419, 31972828/National Natural Science Foundation of China
- 32002419, 31972828/National Natural Science Foundation of China
- 2018YFD0900302-8/National Key Research and Development Program of China
- 2018YFD0900302-8/National Key Research and Development Program of China
- 2018YFD0900302-8/National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

