Circadian clock organization in the retina: From clock components to rod and cone pathways and visual function
- PMID: 36503722
- PMCID: PMC10164718
- DOI: 10.1016/j.preteyeres.2022.101119
Circadian clock organization in the retina: From clock components to rod and cone pathways and visual function
Abstract
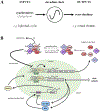
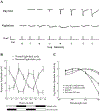
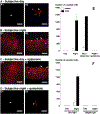
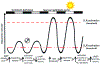
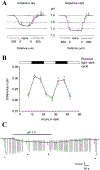
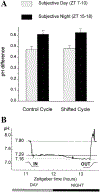
Circadian (24-h) clocks are cell-autonomous biological oscillators that orchestrate many aspects of our physiology on a daily basis. Numerous circadian rhythms in mammalian and non-mammalian retinas have been observed and the presence of an endogenous circadian clock has been demonstrated. However, how the clock and associated rhythms assemble into pathways that support and control retina function remains largely unknown. Our goal here is to review the current status of our knowledge and evaluate recent advances. We describe many previously-observed retinal rhythms, including circadian rhythms of morphology, biochemistry, physiology, and gene expression. We evaluate evidence concerning the location and molecular machinery of the retinal circadian clock, as well as consider findings that suggest the presence of multiple clocks. Our primary focus though is to describe in depth circadian rhythms in the light responses of retinal neurons with an emphasis on clock control of rod and cone pathways. We examine evidence that specific biochemical mechanisms produce these daily light response changes. We also discuss evidence for the presence of multiple circadian retinal pathways involving rhythms in neurotransmitter activity, transmitter receptors, metabolism, and pH. We focus on distinct actions of two dopamine receptor systems in the outer retina, a dopamine D4 receptor system that mediates circadian control of rod/cone gap junction coupling and a dopamine D1 receptor system that mediates non-circadian, light/dark adaptive regulation of gap junction coupling between horizontal cells. Finally, we evaluate the role of circadian rhythmicity in retinal degeneration and suggest future directions for the field of retinal circadian biology.
Keywords: Adenosine; Circadian clock; Circadian rhythmicity; Dopamine; Electrical synapses; Energy metabolism; Gap junctions; Melatonin; Retina; pH.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures

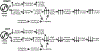
References
-
- Ahmed J, Braun RD, Dunn R Jr., Linsenmeier RA, 1993. Oxygen distribution in the macaque retina. Invest. Ophthalmol. Vis. Sci 34, 516–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

