Establishment of multi-stage intravenous self-administration paradigms in mice
- PMID: 36503898
- PMCID: PMC9742147
- DOI: 10.1038/s41598-022-24740-2
Establishment of multi-stage intravenous self-administration paradigms in mice
Abstract
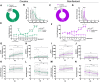
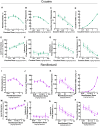
Genetically tractable animal models provide needed strategies to resolve the biological basis of drug addiction. Intravenous self-administration (IVSA) is the gold standard for modeling psychostimulant and opioid addiction in animals, but technical limitations have precluded the widespread use of IVSA in mice. Here, we describe IVSA paradigms for mice that capture the multi-stage nature of the disorder and permit predictive modeling. In these paradigms, C57BL/6J mice with long-standing indwelling jugular catheters engaged in cocaine- or remifentanil-associated lever responding that was fixed ratio-dependent, dose-dependent, extinguished by withholding the drug, and reinstated by the presentation of drug-paired cues. The application of multivariate analysis suggested that drug taking in both paradigms was a function of two latent variables we termed incentive motivation and discriminative control. Machine learning revealed that vulnerability to drug seeking and relapse were predicted by a mouse's a priori response to novelty, sensitivity to drug-induced locomotion, and drug-taking behavior. The application of these behavioral and statistical-analysis approaches to genetically-engineered mice will facilitate the identification of neural circuits driving addiction susceptibility and relapse and focused therapeutic development.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Hedegaard H, Bastian BA, Trinidad JP, Spencer M, Warner M. Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. Natl. Vital Stat. Rep. 2018;69:1–13. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

