A performance comparison of two (electro) chemiluminescence immunoassays for detection and quantitation of serum anti-spike antibodies according to SARS-CoV-2 vaccination and infections status
- PMID: 36504019
- PMCID: PMC9877996
- DOI: 10.1002/jmv.28397
A performance comparison of two (electro) chemiluminescence immunoassays for detection and quantitation of serum anti-spike antibodies according to SARS-CoV-2 vaccination and infections status
Abstract
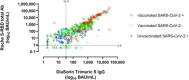
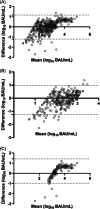
The information provided by SARS-CoV-2 spike (S)-targeting immunoassays can be instrumental in clinical-decision making. We compared the performance of the Elecsys® Anti-SARS-CoV-2 S assay (Roche Diagnostics) and the LIAISON® SARS-CoV-2 TrimericS IgG assay (DiaSorin) using a total of 1176 sera from 797 individuals, of which 286 were from vaccinated-SARS-CoV-2/experienced (Vac-Ex), 581 from vaccinated/naïve (Vac-N), 147 from unvaccinated/experienced (Unvac-Ex), and 162 from unvaccinated/naïve (Unvac-N) individuals. The Roche assay returned a higher number of positive results (907 vs. 790; p = 0.45; overall sensitivity: 89.3% vs. 77.6%). The concordance between results provided by the two immunoassays was higher for sera from Vac-N (ϰ: 0.58; interquartile ranges [IQR]: 0.50-0.65) than for sera from Vac-Ex (ϰ: 0.19; IQR: -0.14 to 0.52) or Unvac-Ex (ϰ: 0.18; IQR: 0.06-0.30). Discordant results occurred more frequently among sera from Unvac-Ex (34.7%) followed by Vac-N (14.6%) and Vac-Ex (2.7%). Antibody levels quantified by both immunoassays were not significantly different when <250 (p = 0.87) or <1000 BAU/ml (p = 0.13); in contrast, for sera ≥1000 BAU/ml, the Roche assay returned significantly higher values than the DiaSorin assay (p < 0.008). Neutralizing antibody titers (NtAb) were measured in 127 sera from Vac-Ex or Vac-N using a S-pseudotyped virus neutralization assay of Wuhan-Hu-1, Omicron BA.1, and Omicron BA.2. The correlation between antibody levels and NtAb titers was higher for sera from Vac-N than those from Vac-Ex, irrespective of the (sub)variant considered. In conclusion, neither qualitative nor quantitative results returned by both immunoassays are interchangeable. The performance of both assays was found to be greatly influenced by the vaccination and SARS-CoV-2 infection status of individuals.
Keywords: DiaSorin immunoassay; Roche immunoassay; S-trimeric antibodies; SARS-CoV-2; antibodies; neutralizing antibodies; receptor binding domain antibodies.
© 2022 The Authors. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Antibody-dependent NK-cell and neutralizing antibody responses against the Spike protein of Wuhan-Hu-1 and Omicron BA.1 SARS-CoV-2 variants in vaccinated experienced and vaccinated naïve individuals.J Med Virol. 2023 Jul;95(7):e28900. doi: 10.1002/jmv.28900. J Med Virol. 2023. PMID: 37403730
-
Cumulative incidence of SARS-CoV-2 infection in the general population of the Valencian Community (Spain) after the surge of the Omicron BA.1 variant.J Med Virol. 2023 Jan;95(1):e28284. doi: 10.1002/jmv.28284. Epub 2022 Nov 11. J Med Virol. 2023. PMID: 36333837 Free PMC article.
-
Inference of SARS-CoV-2 spike-binding neutralizing antibody titers in sera from hospitalized COVID-19 patients by using commercial enzyme and chemiluminescent immunoassays.Eur J Clin Microbiol Infect Dis. 2021 Mar;40(3):485-494. doi: 10.1007/s10096-020-04128-8. Epub 2021 Jan 6. Eur J Clin Microbiol Infect Dis. 2021. PMID: 33404891 Free PMC article.
-
Systematic Review of Diagnostic Accuracy of DiaSorin Liaison SARS-CoV-2 Antigen Immunoassay.EJIFCC. 2022 Aug 8;33(2):94-104. eCollection 2022 Aug. EJIFCC. 2022. PMID: 36313904 Free PMC article. Review.
-
Advancing Luciferase-Based Antibody Immunoassays to Next-Generation Mix and Read Testing.Biosensors (Basel). 2023 Feb 21;13(3):303. doi: 10.3390/bios13030303. Biosensors (Basel). 2023. PMID: 36979515 Free PMC article. Review.
Cited by
-
Delayed but successful development of immune memory against SARS-COV-2 after B cell-depleting monotherapy.Infection. 2025 May 7. doi: 10.1007/s15010-025-02544-6. Online ahead of print. Infection. 2025. PMID: 40332719
-
Dynamics of humoral and cellular response to three doses of anti-SARS-CoV-2 BNT162b2 vaccine in patients with hematological malignancies and older subjects.Front Immunol. 2024 Jan 26;14:1221587. doi: 10.3389/fimmu.2023.1221587. eCollection 2023. Front Immunol. 2024. PMID: 38343436 Free PMC article.
-
SARS-CoV-2-reactive antibody waning, booster effect and breakthrough SARS-CoV-2 infection in hematopoietic stem cell transplant and cell therapy recipients at one year after vaccination.Bone Marrow Transplant. 2023 May;58(5):567-580. doi: 10.1038/s41409-023-01946-0. Epub 2023 Feb 28. Bone Marrow Transplant. 2023. PMID: 36854892 Free PMC article.
-
Estimating SARS-CoV-2 Omicron XBB.1.5 Spike-Directed Functional Antibody Levels From an Anti-Receptor Binding Domain Wuhan-Hu-1-Based Commercial Immunoassay Results.J Med Virol. 2025 Jan;97(1):e70130. doi: 10.1002/jmv.70130. J Med Virol. 2025. PMID: 39812228 Free PMC article.
-
Vaccine-induced SARS-CoV-2 antibody response: the comparability of S1-specific binding assays depends on epitope and isotype discrimination.Front Immunol. 2023 Oct 26;14:1257265. doi: 10.3389/fimmu.2023.1257265. eCollection 2023. Front Immunol. 2023. PMID: 37965324 Free PMC article.
References
-
- Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nature Med. 2021;27:1205‐1211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

