Allograft rejection following immune checkpoint inhibitors in solid organ transplant recipients: A safety analysis from a literature review and a pharmacovigilance system
- PMID: 36504294
- PMCID: PMC10028127
- DOI: 10.1002/cam4.5394
Allograft rejection following immune checkpoint inhibitors in solid organ transplant recipients: A safety analysis from a literature review and a pharmacovigilance system
Abstract
Aim: This study aimed to systematically characterize transplant rejection after immune checkpoint inhibitors (ICIs) initiation in solid organ transplant recipients (SOTRs).
Methods: Data were extracted from the US FDA Adverse Event Reporting System (FAERS) database and case reports in the literature. Disproportionality analysis including information component and reported odds ratio (ROR) was performed to access potential risk signals.
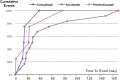
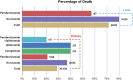
Results: A total of 168 patients with transplant rejection after ICIs usage were identified in the FAERS database, and 89 cases were identified in the literature review. ICIs were significantly associated with transplant rejection (ROR025 : 2.2). A strong risk signal was found for combination therapy with pembrolizumab and ipilimumab compared to monotherapy.
Conclusion: Immune checkpoint inhibitors were significantly associated with transplant rejection in SOTRs.
Keywords: adverse event reporting system; immune checkpoint inhibitors; solid organ transplant; transplant rejection.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures
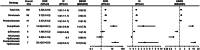

Similar articles
-
Hepatic failure associated with immune checkpoint inhibitors: An analysis of the Food and Drug Administration Adverse Event Reporting System database.Cancer Med. 2023 Apr;12(8):9167-9174. doi: 10.1002/cam4.5655. Epub 2023 Feb 3. Cancer Med. 2023. PMID: 36734333 Free PMC article.
-
Metabolic and Nutritional Disorders Following the Administration of Immune Checkpoint Inhibitors: A Pharmacovigilance Study.Front Endocrinol (Lausanne). 2022 Jan 25;12:809063. doi: 10.3389/fendo.2021.809063. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35145482 Free PMC article.
-
Transplant rejections associated with immune checkpoint inhibitors: A pharmacovigilance study and systematic literature review.Eur J Cancer. 2021 May;148:36-47. doi: 10.1016/j.ejca.2021.01.038. Epub 2021 Mar 12. Eur J Cancer. 2021. PMID: 33721705
-
The Safety and Efficacy of Checkpoint Inhibitors in Transplant Recipients: A Case Series and Systematic Review of Literature.Oncologist. 2020 Jun;25(6):505-514. doi: 10.1634/theoncologist.2019-0659. Epub 2020 Feb 11. Oncologist. 2020. PMID: 32043699 Free PMC article.
-
Renal toxicities in immune checkpoint inhibitors with or without chemotherapy: An observational, retrospective, pharmacovigilance study leveraging US FARES database.Cancer Med. 2021 Dec;10(24):8754-8762. doi: 10.1002/cam4.4343. Epub 2021 Nov 29. Cancer Med. 2021. PMID: 34845857 Free PMC article.
Cited by
-
Outcomes of Solid Organ Transplant Recipients With Advanced Cancers Receiving Immune Checkpoint Inhibitors: A Systematic Review and Individual Participant Data Meta-Analysis.JAMA Oncol. 2025 Jun 22:e252374. doi: 10.1001/jamaoncol.2025.2374. Online ahead of print. JAMA Oncol. 2025. PMID: 40545616
-
Unforeseen Complications of Pembrolizumab in Breast Reconstruction Post-Mastectomy.Eur J Case Rep Intern Med. 2024 Jul 1;11(7):004675. doi: 10.12890/2024_004675. eCollection 2024. Eur J Case Rep Intern Med. 2024. PMID: 38984194 Free PMC article.
-
Impact of exosomes derived from adipose stem cells on lymphocyte proliferation and phenotype in mouse skin grafts.Extracell Vesicles Circ Nucl Acids. 2025 Mar 7;6(1):141-157. doi: 10.20517/evcna.2024.52. eCollection 2025. Extracell Vesicles Circ Nucl Acids. 2025. PMID: 40206795 Free PMC article.
-
Immune Checkpoint Inhibitors and Allograft Rejection Risk: Emerging Evidence Regarding Their Use in Kidney Transplant Recipients.J Clin Med. 2025 Jul 20;14(14):5152. doi: 10.3390/jcm14145152. J Clin Med. 2025. PMID: 40725844 Free PMC article. Review.
-
Fine-tuning tumor- and allo-immunity: advances in the use of immune checkpoint inhibitors in kidney transplant recipients.Clin Kidney J. 2024 Mar 9;17(4):sfae061. doi: 10.1093/ckj/sfae061. eCollection 2024 Apr. Clin Kidney J. 2024. PMID: 38606169 Free PMC article. Review.
References
-
- Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80(2Suppl):S254‐S264. - PubMed
-
- Rama I, Grinyó JM. Malignancy after renal transplantation: the role of immunosuppression. Nat Rev Nephrol. 2010;6(9):511‐519. - PubMed
-
- Hélène B, Gilles Q, Renaud D, et al. Immune‐checkpoint inhibitors to treat cancers in specific immunocompromised populations: a critical review. Expert Rev Antic. 2018;18(10):981‐989. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

