Influenza Vaccine Effectiveness Against Influenza A(H3N2)-Related Illness in the United States During the 2021-2022 Influenza Season
- PMID: 36504336
- PMCID: PMC10893961
- DOI: 10.1093/cid/ciac941
Influenza Vaccine Effectiveness Against Influenza A(H3N2)-Related Illness in the United States During the 2021-2022 Influenza Season
Abstract
Background: In the United States, influenza activity during the 2021-2022 season was modest and sufficient enough to estimate influenza vaccine effectiveness (VE) for the first time since the beginning of the coronavirus disease 2019 pandemic. We estimated influenza VE against laboratory-confirmed outpatient acute illness caused by predominant A(H3N2) viruses.
Methods: Between October 2021 and April 2022, research staff across 7 sites enrolled patients aged ≥6 months seeking outpatient care for acute respiratory illness with cough. Using a test-negative design, we assessed VE against influenza A(H3N2). Due to strong correlation between influenza and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination, participants who tested positive for SARS-CoV-2 were excluded from VE estimations. Estimates were adjusted for site, age, month of illness, race/ethnicity, and general health status.
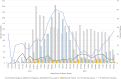
Results: Among 6260 participants, 468 (7%) tested positive for influenza only, including 440 (94%) for A(H3N2). All 206 sequenced A(H3N2) viruses were characterized as belonging to genetic group 3C.2a1b subclade 2a.2, which has antigenic differences from the 2021-2022 season A(H3N2) vaccine component that belongs to clade 3C.2a1b subclade 2a.1. After excluding 1948 SARS-CoV-2-positive patients, 4312 patients were included in analyses of influenza VE; 2463 (57%) were vaccinated against influenza. Effectiveness against A(H3N2) for all ages was 36% (95% confidence interval, 20%-49%) overall.
Conclusions: Influenza vaccination in 2021-2022 provided protection against influenza A(H3N2)-related outpatient visits among young persons.
Keywords: case control; influenza; test-negative; vaccination.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2022.
Conflict of interest statement
Potential conflicts of interest. A. F. reports institutional grant support for research from Gilead, GlaxoSmithKline, Moderna, CDC, and Pfizer, unrelated to this work. C. G. G. reports consulting fees from Merck, Pfizer, and Sanofi Pasteur, and institutional grant support from the Agency for Healthcare Research and Quality, Campbell Alliance/Syneos Health, Food and Drug Administration, CDC, Sanofi, and National Institutes of Health. E. T. M. reports institutional grant support from Merck. A. S. M. reports personal fees from Sanofi and nonfinancial support from Seqirus. M. P. N. reports unrelated institutional grant support and personal fees from Merck Sharp & Dohme and institutional investigator-initiated grant support from Sanofi Pasteur. S. Y. T. reports institutional grant support from Pfizer and GlaxoSmithKline, unrelated to this work. M. G. reports CDC-BSWH US Flu VE Network HAIVEN grant studies, Synergy contract study, CDC-Abt HCP FluVax randomized controlled trial and RECOVER-PROTECT cohort studies, CDC-Vanderbilt IVY-3 and IVY-4 studies, and CDC-Westat VISION COVID/Flu VE Study, unrelated to this work.
Figures


References
-
- Centers for Disease Control and Prevention . 2021–2022 U.S. flu season: preliminary in-season burden estimates. 2022. Available at: https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm. Accessed 23 August 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

