Current Progress on Methods and Technologies for Catalytic Methane Activation at Low Temperatures
- PMID: 36504369
- PMCID: PMC9929156
- DOI: 10.1002/advs.202204566
Current Progress on Methods and Technologies for Catalytic Methane Activation at Low Temperatures
Abstract
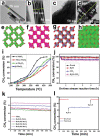
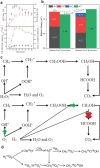
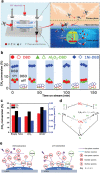
Methane (CH4 ) is an attractive energy source and important greenhouse gas. Therefore, from the economic and environmental point of view, scientists are working hard to activate and convert CH4 into various products or less harmful gas at low-temperature. Although the inert nature of CH bonds requires high dissociation energy at high temperatures, the efforts of researchers have demonstrated the feasibility of catalysts to activate CH4 at low temperatures. In this review, the efficient catalysts designed to reduce the CH4 oxidation temperature and improve conversion efficiencies are described. First, noble metals and transition metal-based catalysts are summarized for activating CH4 in temperatures ranging from 50 to 500 °C. After that, the partial oxidation of CH4 at relatively low temperatures, including thermocatalysis in the liquid phase, photocatalysis, electrocatalysis, and nonthermal plasma technologies, is briefly discussed. Finally, the challenges and perspectives are presented to provide a systematic guideline for designing and synthesizing the highly efficient catalysts in the complete/partial oxidation of CH4 at low temperatures.
Keywords: CH bond activation; low-temperature; methane activation; noble metal-based catalysts; transition metal-based catalysts.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














Similar articles
-
Single Atom Catalysts for Selective Methane Oxidation to Oxygenates.ACS Nano. 2022 Jun 28;16(6):8557-8618. doi: 10.1021/acsnano.2c02464. Epub 2022 May 31. ACS Nano. 2022. PMID: 35638813 Review.
-
Designing catalysts for functionalization of unactivated C-H bonds based on the CH activation reaction.Acc Chem Res. 2012 Jun 19;45(6):885-98. doi: 10.1021/ar200250r. Epub 2012 Apr 6. Acc Chem Res. 2012. PMID: 22482496
-
Recent progress of catalytic methane combustion over transition metal oxide catalysts.Front Chem. 2022 Aug 8;10:959422. doi: 10.3389/fchem.2022.959422. eCollection 2022. Front Chem. 2022. PMID: 36003612 Free PMC article. Review.
-
Palladium Catalysts for Methane Oxidation: Old Materials, New Challenges.Acc Chem Res. 2024 Jan 2;57(1):23-36. doi: 10.1021/acs.accounts.3c00454. Epub 2023 Dec 15. Acc Chem Res. 2024. PMID: 38099741
-
Activation and catalytic transformation of methane under mild conditions.Chem Soc Rev. 2022 Jan 4;51(1):376-423. doi: 10.1039/d1cs00783a. Chem Soc Rev. 2022. PMID: 34904592 Review.
References
-
- Wang Y., Arandiyan H., Scott J., Akia M., Dai H., Deng J., Aguey‐Zinsou K.‐F., Amal R., ACS Catal. 2016, 6, 6935.
-
- Wang Y., Hu P., Yang J., Zhu Y.‐A., Chen D., Chem. Soc. Rev. 2021, 50, 4299. - PubMed
-
- Xiao Y., Li J., Wang C., Zhong F., Zheng Y., Jiang L., Catal. Sci. Technol. 2021, 11, 836.
-
- Lambert C. K., Nat. Catal. 2019, 2, 554.
-
- Yang J., Chang Y., Dai W., Wu G., Guan N., Li L., Appl. Catal., B 2018, 236, 404.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
