Familial atrial fibrillation mutation M1875T-SCN5A increases early sodium current and dampens the effect of flecainide
- PMID: 36504385
- PMCID: PMC10062360
- DOI: 10.1093/europace/euac218
Familial atrial fibrillation mutation M1875T-SCN5A increases early sodium current and dampens the effect of flecainide
Abstract
Aims: Atrial fibrillation (AF) is the most common cardiac arrhythmia. Pathogenic variants in genes encoding ion channels are associated with familial AF. The point mutation M1875T in the SCN5A gene, which encodes the α-subunit of the cardiac sodium channel Nav1.5, has been associated with increased atrial excitability and familial AF in patients.
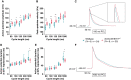
Methods and results: We designed a new murine model carrying the Scn5a-M1875T mutation enabling us to study the effects of the Nav1.5 mutation in detail in vivo and in vitro using patch clamp and microelectrode recording of atrial cardiomyocytes, optical mapping, electrocardiogram, echocardiography, gravimetry, histology, and biochemistry. Atrial cardiomyocytes from newly generated adult Scn5a-M1875T+/- mice showed a selective increase in the early (peak) cardiac sodium current, larger action potential amplitude, and a faster peak upstroke velocity. Conduction slowing caused by the sodium channel blocker flecainide was less pronounced in Scn5a-M1875T+/- compared to wildtype atria. Overt hypertrophy or heart failure in Scn5a-M1875T+/- mice could be excluded.
Conclusion: The Scn5a-M1875T point mutation causes gain-of-function of the cardiac sodium channel. Our results suggest increased atrial peak sodium current as a potential trigger for increased atrial excitability.
Keywords: Atrial electrophysiology; Channelopathy; Sodium channel; Sodium channel blocker.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: L.F. has received institutional research grants from governmental and charity funding agencies and several biomedical companies. P.K. has received research support from several drug and device companies active in atrial fibrillation and has received honoraria from several such companies in the past. L.F. and P.K. are listed as inventors on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 015140571, Markers for Atrial Fibrillation WO 2016012783).
Figures





References
-
- Fabritz L, Crijns H, Guasch E, Goette A, Hausler KG, Kotecha Det al. . Dynamic risk assessment to improve quality of care in patients with atrial fibrillation: the 7th AFNET/EHRA consensus conference. Europace 2021;23:329–44. - PubMed
-
- Fabritz L, Guasch E, Antoniades C, Bardinet I, Benninger G, Betts TRet al. . Expert consensus document: defining the major health modifiers causing atrial fibrillation: a roadmap to underpin personalized prevention and treatment. Nat Rev Cardiol 2016;13:230–7. - PubMed
-
- Makiyama T, Akao M, Shizuta S, Doi T, Nishiyama K, Oka Yet al. . A novel SCN5A gain-of-function mutation M1875T associated with familial atrial fibrillation. J Am Coll Cardiol 2008;52:1326–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

