Dose-dependent effects of esketamine on brain activity in awake mice: A BOLD phMRI study
- PMID: 36504448
- PMCID: PMC9743060
- DOI: 10.1002/prp2.1035
Dose-dependent effects of esketamine on brain activity in awake mice: A BOLD phMRI study
Abstract
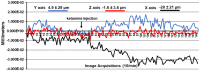
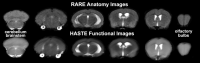
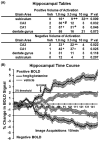
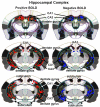
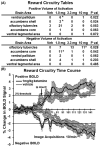
Pharmacological magnetic resonance imaging (phMRI) is a noninvasive method used to evaluate neural circuitry involved in the behavioral effects of drugs like ketamine, independent of their specific biochemical mechanism. The study was designed to evaluate the immediate effect of esketamine, the S-isomer of (±) ketamine on brain activity in awake mice using blood oxygenation level dependent (BOLD) imaging. It was hypothesized the prefrontal cortex, hippocampus, and brain areas associated with reward and motivation would show a dose-dependent increase in brain activity. Mice were given vehicle, 1.0, 3.3, or 10 mg/kg esketamine I.P. and imaged for 10 min post-treatment. Data for each treatment were registered to a 3D MRI mouse brain atlas providing site-specific information on 134 different brain areas. There was a global change in brain activity for both positive and negative BOLD signal affecting over 50 brain areas. Many areas showed a dose-dependent decrease in positive BOLD signal, for example, cortex, hippocampus, and thalamus. The most common profile when comparing the three doses was a U-shape with the 3.3 dose having the lowest change in signal. At 1.0 mg/kg there was a significant increase in positive BOLD in forebrain areas and hippocampus. The anticipated dose-dependent increase in BOLD was not realized; instead, the lowest dose of 1.0 mg/kg had the greatest effect on brain activity. The prefrontal cortex and hippocampus were significantly activated corroborating previous imaging studies in humans and animals. The unexpected sensitivity to the 1.0 mg/kg dose of esketamine could be explained by imaging in fully awake mice without the confound of anesthesia and/or its greater affinity for the N-methyl-d-aspartate receptor (NMDAR) receptor than (±) ketamine.
Keywords: NMDA; depression; hippocampus; phMRI; prefrontal cortex.
© 2022 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Figures

References
-
- Chow W, Doane MJ, Sheehan JP, Alphs L, Le H. Economic burden among patients with major depressive disorder: an analysis of healthcare resource use, work productivity, and direct and indirect costs by depression severity. Am J Manag Care. 2019;23:262‐270.
-
- Malhi GS, Bell E, Singh AB, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: major depression summary. Bipolar Disord. 2020;22:788‐804. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

