Development of Epstein-Barr virus-associated gastric cancer: Infection, inflammation, and oncogenesis
- PMID: 36504553
- PMCID: PMC9730441
- DOI: 10.3748/wjg.v28.i44.6249
Development of Epstein-Barr virus-associated gastric cancer: Infection, inflammation, and oncogenesis
Abstract
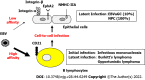
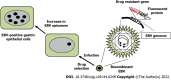
Epstein-Barr virus (EBV)-associated gastric cancer (EBVaGC) cells originate from a single-cell clone infected with EBV. However, more than 95% of patients with gastric cancer have a history of Helicobacter pylori (H. pylori) infection, and H. pylori is a major causative agent of gastric cancer. Therefore, it has long been argued that H. pylori infection may affect the development of EBVaGC, a subtype of gastric cancer. Atrophic gastrointestinal inflammation, a symptom of H. pylori infection, is observed in the gastric mucosa of EBVaGC. Therefore, it remains unclear whether H. pylori infection is a cofactor for gastric carcinogenesis caused by EBV infection or whether H. pylori and EBV infections act independently on gastric cancer formation. It has been reported that EBV infection assists in the onco-genesis of gastric cancer caused by H. pylori infection. In contrast, several studies have reported that H. pylori infection accelerates tumorigenesis initiated by EBV infection. By reviewing both clinical epidemiological and experimental data, we reorganized the role of H. pylori and EBV infections in gastric cancer formation.
Keywords: Coreceptor; Epstein-Barr virus; Epstein-Barr virus-associated gastric cancer; Helicobacter pylori; Inflammation; Oncogenesis.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures

References
-
- Matsusaka K, Funata S, Fukuyo M, Seto Y, Aburatani H, Fukayama M, Kaneda A. Epstein-Barr virus infection induces genome-wide de novo DNA methylation in non-neoplastic gastric epithelial cells. J Pathol. 2017;242:391–399. - PubMed
-
- Matsusaka K, Kaneda A, Nagae G, Ushiku T, Kikuchi Y, Hino R, Uozaki H, Seto Y, Takada K, Aburatani H, Fukayama M. Classification of Epstein-Barr virus-positive gastric cancers by definition of DNA methylation epigenotypes. Cancer Res. 2011;71:7187–7197. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

