Brain arteriovenous malformation in hereditary hemorrhagic telangiectasia: Recent advances in cellular and molecular mechanisms
- PMID: 36504622
- PMCID: PMC9729275
- DOI: 10.3389/fnhum.2022.1006115
Brain arteriovenous malformation in hereditary hemorrhagic telangiectasia: Recent advances in cellular and molecular mechanisms
Abstract
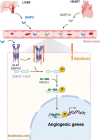
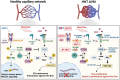
Hereditary hemorrhagic telangiectasia (HHT) is a genetic disorder characterized by vessel dilatation, such as telangiectasia in skin and mucosa and arteriovenous malformations (AVM) in internal organs such as the gastrointestinal tract, lungs, and brain. AVMs are fragile and tortuous vascular anomalies that directly connect arteries and veins, bypassing healthy capillaries. Mutations in transforming growth factor β (TGFβ) signaling pathway components, such as ENG (ENDOGLIN), ACVRL1 (ALK1), and SMAD4 (SMAD4) genes, account for most of HHT cases. 10-20% of HHT patients develop brain AVMs (bAVMs), which can lead to vessel wall rupture and intracranial hemorrhages. Though the main mutations are known, mechanisms leading to AVM formation are unclear, partially due to lack of animal models. Recent mouse models allowed significant advances in our understanding of AVMs. Endothelial-specific deletion of either Acvrl1, Eng or Smad4 is sufficient to induce AVMs, identifying endothelial cells (ECs) as primary targets of BMP signaling to promote vascular integrity. Loss of ALK1/ENG/SMAD4 signaling is associated with NOTCH signaling defects and abnormal arteriovenous EC differentiation. Moreover, cumulative evidence suggests that AVMs originate from venous ECs with defective flow-migration coupling and excessive proliferation. Mutant ECs show an increase of PI3K/AKT signaling and inhibitors of this signaling pathway rescue AVMs in HHT mouse models, revealing new therapeutic avenues. In this review, we will summarize recent advances and current knowledge of mechanisms controlling the pathogenesis of bAVMs, and discuss unresolved questions.
Keywords: ALK1; AVM; BMP; ENG; HHT; SMAD4; endothelial cells.
Copyright © 2022 Drapé, Anquetil, Larrivée and Dubrac.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Overexpression of Activin Receptor-Like Kinase 1 in Endothelial Cells Suppresses Development of Arteriovenous Malformations in Mouse Models of Hereditary Hemorrhagic Telangiectasia.Circ Res. 2020 Oct 9;127(9):1122-1137. doi: 10.1161/CIRCRESAHA.119.316267. Epub 2020 Jul 31. Circ Res. 2020. PMID: 32762495 Free PMC article.
-
SMAD4 Prevents Flow Induced Arteriovenous Malformations by Inhibiting Casein Kinase 2.Circulation. 2018 Nov 20;138(21):2379-2394. doi: 10.1161/CIRCULATIONAHA.118.033842. Circulation. 2018. PMID: 29976569 Free PMC article.
-
Angiopoietin-2 Inhibition Rescues Arteriovenous Malformation in a Smad4 Hereditary Hemorrhagic Telangiectasia Mouse Model.Circulation. 2019 Apr 23;139(17):2049-2063. doi: 10.1161/CIRCULATIONAHA.118.036952. Circulation. 2019. PMID: 30744395 Free PMC article.
-
An update on preclinical models of hereditary haemorrhagic telangiectasia: Insights into disease mechanisms.Front Med (Lausanne). 2022 Sep 29;9:973964. doi: 10.3389/fmed.2022.973964. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36250069 Free PMC article. Review.
-
Hereditary Haemorrhagic Telangiectasia, an Inherited Vascular Disorder in Need of Improved Evidence-Based Pharmaceutical Interventions.Genes (Basel). 2021 Jan 27;12(2):174. doi: 10.3390/genes12020174. Genes (Basel). 2021. PMID: 33513792 Free PMC article. Review.
Cited by
-
Common and distinct circulating microRNAs in four neurovascular disorders.Biochem Biophys Rep. 2025 Aug 2;43:102189. doi: 10.1016/j.bbrep.2025.102189. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40800603 Free PMC article.
-
Current perspectives and trends in the treatment of brain arteriovenous malformations: a review and bibliometric analysis.Front Neurol. 2024 Jan 11;14:1327915. doi: 10.3389/fneur.2023.1327915. eCollection 2023. Front Neurol. 2024. PMID: 38274874 Free PMC article.
-
YAP/TAZ drives Notch and angiogenesis mechanoregulation in silico.NPJ Syst Biol Appl. 2024 Oct 5;10(1):116. doi: 10.1038/s41540-024-00444-3. NPJ Syst Biol Appl. 2024. PMID: 39368976 Free PMC article.
-
Somatic mutations in arteriovenous malformations in hereditary hemorrhagic telangiectasia support a bi-allelic two-hit mutation mechanism of pathogenesis.Am J Hum Genet. 2024 Oct 3;111(10):2283-2298. doi: 10.1016/j.ajhg.2024.08.020. Epub 2024 Sep 18. Am J Hum Genet. 2024. PMID: 39299239 Free PMC article.
-
Defining the Role of Oral Pathway Inhibitors as Targeted Therapeutics in Arteriovenous Malformation Care.Biomedicines. 2024 Jun 11;12(6):1289. doi: 10.3390/biomedicines12061289. Biomedicines. 2024. PMID: 38927496 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

