A combined experimental and molecular simulation study on stress generation phenomena during the Ziegler-Natta polyethylene catalyst fragmentation process
- PMID: 36504732
- PMCID: PMC9680958
- DOI: 10.1039/d2na00406b
A combined experimental and molecular simulation study on stress generation phenomena during the Ziegler-Natta polyethylene catalyst fragmentation process
Abstract
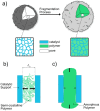
The morphology of particles obtained under different pre-polymerization conditions has been connected to the stress generation mechanism at the polymer/catalyst interface. A combination of experimental characterization techniques and atomistic molecular dynamics simulations allowed a systematic investigation of experimental conditions leading to a certain particle morphology, and hence to a final polymer with specific features. Atomistic models of nascent polymer phases in contact with magnesium dichloride surfaces have been developed and validated. Using these detailed models, in the framework of McKenna's hypothesis, the pressure increase due to the polymerization reaction has been calculated under different conditions and is in good agreement with experimental scenarios. This molecular scale knowledge and the proposed investigation strategy would allow the pre-polymerization conditions to be better defined and the properties of the nascent polymer to be tuned, ensuring proper operability along the whole polymer production process.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Rahimi A. García J. M. Nat. Rev. Chem. 2017;1:0046. doi: 10.1038/s41570-017-0046. - DOI
-
- Soares J. B. P., McKenna T. F. L., Polyolefin Reaction Engineering, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2012, pp. 87–129, ISBN: 978-3-527-31710-3
-
- Zhou Y. Alizadeh A. Liu B. Soares J. B. P. Macromol. React. Eng. 2021;15:2000043. doi: 10.1002/mren.202000043. - DOI
LinkOut - more resources
Full Text Sources

