Simple and sustainable synthesis of perovskite-based optoelectronic material: CsPbBr3 nanocrystals via laser ablation in alcohol
- PMID: 36504746
- PMCID: PMC9680829
- DOI: 10.1039/d2na00596d
Simple and sustainable synthesis of perovskite-based optoelectronic material: CsPbBr3 nanocrystals via laser ablation in alcohol
Abstract
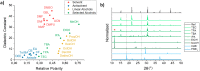
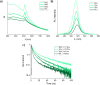
All-inorganic lead halide perovskite nanocrystals (NCs) have shown great potential as emerging semiconducting materials due to their excellent optoelectronic properties. However, syntheses in solution commonly use high temperatures and toxic solvents, which are obstacles for safety and sustainability of the process. In this work, laser ablation in alcohol is proposed as a simple and sustainable, ligand-free, top-down approach to synthesize CsPbBr3 nanocrystals in ambient conditions. The effects of different low boiling point commercial alcohols used as solvents on the optical properties of CsPbBr3 NCs colloidal solutions are investigated. Although in traditional bottom-up synthesis alcohols are usually found to be not appropriate for the synthesis of perovskite NCs, here it is demonstrated that CsPbBr3 orthorhombic nanocrystals with narrow full width half maximum (FWHM < 18 nm), long photoluminescence lifetimes (up to 17.9 ns) and good photoluminescence quantum yield (PLQY up to 15.5%) can be obtained by selecting the dielectric constant and polarity of the alcohol employed for the synthesis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Palabathuni M. Akhil S. Singh R. Mishra N. Charge Transfer in Photoexcited Cesium–Lead Halide Perovskite Nanocrystals: Review of Materials and Applications. ACS Appl. Nano Mater. 2022;5:10097–10117. doi: 10.1021/acsanm.2c01550. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

