phoD-harboring bacterial community composition dominates organic P mineralization under long-term P fertilization in acid purple soil
- PMID: 36504812
- PMCID: PMC9730253
- DOI: 10.3389/fmicb.2022.1045919
phoD-harboring bacterial community composition dominates organic P mineralization under long-term P fertilization in acid purple soil
Abstract
Introduction: A better understanding of the regulatory role of microorganisms on soil phosphorous (P) mobilization is critical for developing sustainable fertilization practices and reducing P resource scarcity. The phoD genes regulate soil organic P (Po) mobilization.
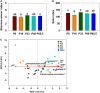
Methods: Based on the long-term P application experiments in acid purple soil of maize system in Southwest China (started in 2010), the experiment included five P levels: 0, 16, 33, 49, and 65.5 kg P hm-2 (P0, P16, P33, P49, and P65.5, respectively). The molecular speciation of organic P in soil was determined by 31P-nuclear magnetic resonance (NMR), high-throughput sequencing technology, and real-time qPCR were used to analyze the bacterial community and abundance of phoD-harboring bacterial genes, exploring the bacterial community and abundance characteristics of phoD gene and its relationship with the forms of Po and alkaline phosphatase (ALP) activity in the soil.
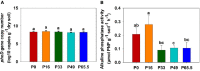
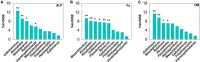
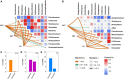
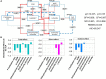
Results: The results showed that the orthophosphate monoesters (OM) were the main Po speciation and varied by P fertilization in acid purple soil. ALP activity decreased as P fertilization increased. Co-occurrence network analysis identified the overall network under five P fertilizations. The keystone taxon base on the network showed that Collimonas, Roseateles, Mesorhizobium, and Cellulomonas positively correlated with both OM and Po. The random forest showed that Cellulomonas, Roseateles, and Rhodoplanes were the key predictors for ALP activity. The keystone taxon was a more important predictor than the dominant taxon for ALP, OM, and Po. The structural equation model (SEM) showed that soil organic matter (SOM), available P (AP), and OM were the main factors influencing the ALP by reshaping phoD-harboring bacteria alpha diversity, community composition, and phoD abundance.
Discussion: The phoD-harboring bacterial community composition especially the keystone taxon rather than alpha diversity and abundance dominated the ALP activity, which could promote P utilization over an intensive agroecosystem. These findings improve the understanding of how long-term gradient fertilization influences the community composition and function of P-solubilizing microorganisms in acid purple soil.
Keywords: alkaline phosphatase; co-occurrence network; keystone taxa; long-term P fertilization; organic P speciation; phoD-harboring bacteria.
Copyright © 2022 Lang, Li, Lakshmanan, Chen and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Anderson M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral J. Ecol. 26 32–46.
-
- Baba Z. A., Hamid B., Sheikh T. A., Alotaibi S. H., El Enshasy H. A., Ansari M. J., et al. (2021). Psychrotolerant Mesorhizobium sp. Isolated from temperate and cold desert regions solubilizes potassium and produces multiple plant growth promoting metabolites. Molecules 26:5758. 10.3390/molecules26195758 - DOI - PMC - PubMed
-
- Bastian M., Heymann S., Jacomy M. (2009). Gephi: An open source software for exploring and manipulating networks. Proc. Int. AAAI Conf. Web Soc. Media 3 361–362. 10.1609/icwsm.v3i1.13937 - DOI
LinkOut - more resources
Full Text Sources

