Insight into aphid mediated Potato Virus Y transmission: A molecular to bioinformatics prospective
- PMID: 36504828
- PMCID: PMC9729956
- DOI: 10.3389/fmicb.2022.1001454
Insight into aphid mediated Potato Virus Y transmission: A molecular to bioinformatics prospective
Abstract
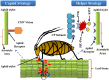
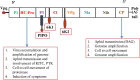
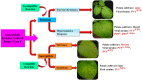
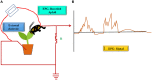
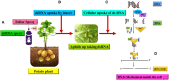
Potato, the world's most popular crop is reported to provide a food source for nearly a billion people. It is prone to a number of biotic stressors that affect yield and quality, out of which Potato Virus Y (PVY) occupies the top position. PVY can be transmitted mechanically and by sap-feeding aphid vectors. The application of insecticide causes an increase in the resistant vector population along with detrimental effects on the environment; genetic resistance and vector-virus control are the two core components for controlling the deadly PVY. Using transcriptomic tools together with differential gene expression and gene discovery, several loci and genes associated with PVY resistance have been widely identified. To combat this virus we must increase our understanding on the molecular response of the PVY-potato plant-aphid interaction and knowledge of genome organization, as well as the function of PVY encoded proteins, genetic diversity, the molecular aspects of PVY transmission by aphids, and transcriptome profiling of PVY infected potato cultivars. Techniques such as molecular and bioinformatics tools can identify and monitor virus transmission. Several studies have been conducted to understand the molecular basis of PVY resistance/susceptibility interactions and their impact on PVY epidemiology by studying the interrelationship between the virus, its vector, and the host plant. This review presents current knowledge of PVY transmission, epidemiology, genome organization, molecular to bioinformatics responses, and its effective management.
Keywords: Potato Virus Y (PVY); aphid; bioinformatics; genome; molecular; potato; transcriptome; vector.
Copyright © 2022 Bhoi, Samal, Majhi, Komal, Mahanta, Pradhan, Saini, Nikhil Raj, Ahmad, Behera and Ashwini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aarts N., Metz M., Holub E., Staskawicz B. J., Daniels M. J., Parker J. E. (1998). Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 95, 10306–10311. 10.1073/pnas.95.17.10306 - DOI - PMC - PubMed
-
- Aleman-Verdaguer M. E., Goudou-Urbino C., Dubern J. B., Beachy R. N. C., Fauquet C. (1997). Analysis of the sequence diversity of the P1, HC, P3, NIb and CP genomic regions of several yam mosaic Potyvirus isolates: implications for the intraspecies molecular diversity of potyviruses. J. Gen. Virol. 78, 1253–1264. 10.1099/0022-1317-78-6-1253 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

