Adenosine reduces sinoatrial node cell action potential firing rate by uncoupling its membrane and calcium clocks
- PMID: 36505046
- PMCID: PMC9730041
- DOI: 10.3389/fphys.2022.977807
Adenosine reduces sinoatrial node cell action potential firing rate by uncoupling its membrane and calcium clocks
Abstract
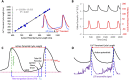
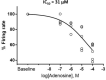
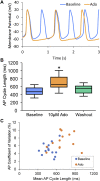
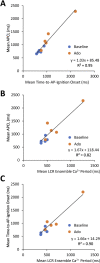
The spontaneous action potential (AP) firing rate of sinoatrial nodal cells (SANC) is regulated by a system of intracellular Ca2+ and membrane ion current clocks driven by Ca2+-calmodulin-activated adenylyl cyclase-protein kinase-A signaling. The mean AP-cycle length (APCL) and APCL variability inform on the effectiveness of clock coupling. Endogenous ATP metabolite adenosine binds to adenosine receptors (A1, A3) that couple to Gi protein-coupled receptors, reducing spontaneous AP firing rate via Gβγ signaling that activates IKAch,Ado. Adenosine also inhibits adenylyl cyclase activity via Gαi signaling, impacting cAMP-mediated protein kinase-A-dependent protein phosphorylation. We hypothesize that in addition to IKAch,Ado activation, adenosine impacts also Ca2+ via Gαi signaling and that both effects reduce AP firing rate by reducing the effectiveness of the Ca2+ and membrane clock coupling. To this end, we measured Ca2+ and membrane potential characteristics in enzymatically isolated single rabbit SANC. 10 µM adenosine substantially increased both the mean APCL (on average by 43%, n = 10) and AP beat-to-beat variability from 5.1 ± 1.7% to 7.2 ± 2.0% (n = 10) measured via membrane potential and 5.0 ± 2.2% to 10.6 ± 5.9% (n = 40) measured via Ca2+ (assessed as the coefficient of variability = SD/mean). These effects were mediated by hyperpolarization of the maximum diastolic membrane potential (membrane clock effect) and suppression of diastolic local Ca2+releases (LCRs) (Ca2+-clock effect): as LCR size distributions shifted to smaller values, the time of LCR occurrence during diastolic depolarization (LCR period) became prolonged, and the ensemble LCR signal became reduced. The tight linear relationship of coupling between LCR period to the APCL in the presence of adenosine "drifted" upward and leftward, i.e. for a given LCR period, APCL was prolonged, becoming non-linear indicating clock uncoupling. An extreme case of uncoupling occurred at higher adenosine concentrations (>100 µM): small stochastic LCRs failed to self-organize and synchronize to the membrane clock, thus creating a failed attempt to generate an AP resulting in arrhythmia and cessation of AP firing. Thus, the effects of adenosine to activate Gβγ and IKACh,Ado and to activate Gαi, suppressing adenylyl cyclase activity, both contribute to the adenosine-induced increase in the mean APCL and APCL variability by reducing the fidelity of clock coupling and AP firing rate.
Keywords: adenosine; calcium release; cardiac arrhythmia; coupled-clock pacemaker system; sarcoplasmic reticulum (SR); sick sinus syndrome; sinoatrial node (SAN); sinus node arrest.
Copyright © 2022 Wirth, Tsutsui, Maltsev and Lakatta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Spontaneous, local diastolic subsarcolemmal calcium releases in single, isolated guinea-pig sinoatrial nodal cells.PLoS One. 2017 Sep 25;12(9):e0185222. doi: 10.1371/journal.pone.0185222. eCollection 2017. PLoS One. 2017. PMID: 28945810 Free PMC article.
-
cAMP-Dependent Signaling Restores AP Firing in Dormant SA Node Cells via Enhancement of Surface Membrane Currents and Calcium Coupling.Front Physiol. 2021 Apr 9;12:596832. doi: 10.3389/fphys.2021.596832. eCollection 2021. Front Physiol. 2021. PMID: 33897445 Free PMC article.
-
New evidence for coupled clock regulation of the normal automaticity of sinoatrial nodal pacemaker cells: bradycardic effects of ivabradine are linked to suppression of intracellular Ca²⁺ cycling.J Mol Cell Cardiol. 2013 Sep;62:80-9. doi: 10.1016/j.yjmcc.2013.04.026. Epub 2013 May 5. J Mol Cell Cardiol. 2013. PMID: 23651631 Free PMC article.
-
Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) Regulates Basal Cardiac Pacemaker Function: Pros and Cons.Cells. 2024 Dec 25;14(1):3. doi: 10.3390/cells14010003. Cells. 2024. PMID: 39791704 Free PMC article. Review.
-
Unique Ca2+-Cycling Protein Abundance and Regulation Sustains Local Ca2+ Releases and Spontaneous Firing of Rabbit Sinoatrial Node Cells.Int J Mol Sci. 2018 Jul 25;19(8):2173. doi: 10.3390/ijms19082173. Int J Mol Sci. 2018. PMID: 30044420 Free PMC article. Review.
Cited by
-
Mitochondrial dysfunction is a key link involved in the pathogenesis of sick sinus syndrome: a review.Front Cardiovasc Med. 2024 Oct 29;11:1488207. doi: 10.3389/fcvm.2024.1488207. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39534498 Free PMC article. Review.
References
-
- Axsom J. E., Nanavati A. P., Rutishauser C. A., Bonin J. E., Moen J. M., Lakatta E. G. (2020). Acclimation to a thermoneutral environment abolishes age-associated alterations in heart rate and heart rate variability in conscious, unrestrained mice. Geroscience 42, 217–232. 10.1007/s11357-019-00126-7 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

