Multisystem immune-related adverse events due to toripalimab: Two cases-based review
- PMID: 36505392
- PMCID: PMC9732722
- DOI: 10.3389/fcvm.2022.1036603
Multisystem immune-related adverse events due to toripalimab: Two cases-based review
Abstract
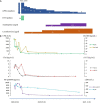
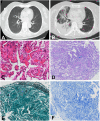
Immune checkpoint inhibitors (ICIs) have significantly improved the survival of patients with advanced tumors. However, immune-related adverse events (irAEs) caused by ICIs, especially high-grade irAEs, are of growing concern. High-grade multisystem irAEs due to toripalimab, a programmed cell death-1 (PD-1) inhibitor, have been rarely reported. Two patients with malignant metastatic tumors were treated with anti-PD-1 immunotherapy. However, both patients developed high-grade multisystem irAEs based on myocarditis, with chest discomfort and malaise as the main clinical manifestation. Both patients had an elevation of cardiac enzymes, abnormal electrocardiography and left ventricular wall motion. Patient 2 was also diagnosed with organizing pneumonia. Immunotherapy was suspended. High-dose intravenous methylprednisolone was immediately initiated. The patients' symptoms were significantly relieved in a short period of time. Immunosuppressants were discontinued at the 6th month follow-up in patient 1 without relapse. However, patient 2 was lost to follow up due to financial reasons. To the best of our knowledge, this is the first report regarding ICI-associated myocarditis-pneumonia due to toripalimab, indicating the significance of early recognition and management of high-grade multisystem irAEs in clinical practice.
Keywords: PD-1; immune checkpoint inhibitors; immune-related adverse events; myocarditis; pneumonia.
Copyright © 2022 Chen, Chen, Xie, Liu and Hong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

