Impact of dietary vitamin D on immunoregulation and disease pathology in lupus-prone NZB/W F1 mice
- PMID: 36505422
- PMCID: PMC9730823
- DOI: 10.3389/fimmu.2022.933191
Impact of dietary vitamin D on immunoregulation and disease pathology in lupus-prone NZB/W F1 mice
Abstract
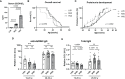
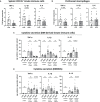
Vitamin D (VD) deficiency is a highly prevalent worldwide phenomenon and is extensively discussed as a risk factor for the development of systemic lupus erythematosus (SLE) and other immune-mediated diseases. In addition, it is now appreciated that VD possesses multiple immunomodulatory effects. This study aims to explore the impact of dietary VD intake on lupus manifestation and pathology in lupus-prone NZB/W F1 mice and identify the underlying immunological mechanisms modulated by VD. Here, we show that low VD intake accelerates lupus progression, reflected in reduced overall survival and an earlier onset of proteinuria, as well higher concentrations of anti-double-stranded DNA autoantibodies. This unfavorable effect gained statistical significance with additional low maternal VD intake during the prenatal period. Among examined immunological effects, we found that low VD intake consistently hampered the adoption of a regulatory phenotype in lymphocytes, significantly reducing both IL-10-expressing and regulatory CD4+ T cells. This goes along with a mildly decreased frequency of IL-10-expressing B cells. We did not observe consistent effects on the phenotype and function of innate immune cells, including cytokine production, costimulatory molecule expression, and phagocytic capacity. Hence, our study reveals that low VD intake promotes lupus pathology, likely via the deviation of adaptive immunity, and suggests that the correction of VD deficiency might not only exert beneficial functions by preventing osteoporosis but also serve as an important module in prophylaxis and as an add-on in the treatment of lupus and possibly other immune-mediated diseases. Further research is required to determine the most appropriate dosage, as too-high VD serum levels may also induce adverse effects, possibly also on lupus pathology.
Keywords: SLE; autoimmune; autoimmunity; choleacliferol; diet; lupus; mice; vitamin D.
Copyright © 2022 Kraemer, Schäfer, Sprenger, Sehnert, Williams, Luo, Riechert, Al-Kayyal, Dumortier, Fauny, Winter, Heim, Hofmann, Herrmann, Heine, Voll and Chevalier.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
T cell cytokine imbalance towards production of IFN-gamma and IL-10 in NZB/W F1 lupus-prone mice is associated with autoantibody levels and nephritis.Scand J Rheumatol. 2006 May-Jun;35(3):209-16. doi: 10.1080/03009740500417791. Scand J Rheumatol. 2006. PMID: 16766368
-
Soluble human CD83 ameliorates lupus in NZB/W F1 mice.Immunobiology. 2013 Nov;218(11):1411-5. doi: 10.1016/j.imbio.2013.06.002. Epub 2013 Jun 17. Immunobiology. 2013. PMID: 23886695
-
Vitamin D status and its relationship with systemic lupus erythematosus as a determinant and outcome of disease activity.Horm Mol Biol Clin Investig. 2019 Apr 3;38(3):/j/hmbci.2019.38.issue-3/hmbci-2018-0064/hmbci-2018-0064.xml. doi: 10.1515/hmbci-2018-0064. Horm Mol Biol Clin Investig. 2019. PMID: 30943171
-
Dual Role of Interleukin-10 in Murine NZB/W F1 Lupus.Int J Mol Sci. 2021 Jan 29;22(3):1347. doi: 10.3390/ijms22031347. Int J Mol Sci. 2021. PMID: 33572870 Free PMC article.
-
Immunomodulatory effects of H.P. Acthar Gel on B cell development in the NZB/W F1 mouse model of systemic lupus erythematosus.Lupus. 2014 Jul;23(8):802-12. doi: 10.1177/0961203314531840. Epub 2014 Apr 23. Lupus. 2014. PMID: 24759631
Cited by
-
The immunomodulatory potential of vitamin D on Th17 lymphocytes in systemic lupus erythematosus - a literature review.Med Pharm Rep. 2025 Jan;98(1):13-20. doi: 10.15386/mpr-2752. Epub 2025 Jan 31. Med Pharm Rep. 2025. PMID: 39949914 Free PMC article. Review.
-
Characterization of Dendritic Cells and Myeloid-Derived Suppressor Cells Expressing Major Histocompatibility Complex Class II in Secondary Lymphoid Organs in Systemic Lupus Erythematosus-Prone Mice.Int J Mol Sci. 2024 Dec 19;25(24):13604. doi: 10.3390/ijms252413604. Int J Mol Sci. 2024. PMID: 39769367 Free PMC article.
References
-
- Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheumatism (1998) 41(7):1241–50. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

