Modulating macrophage function to reinforce host innate resistance against Mycobacterium avium complex infection
- PMID: 36505429
- PMCID: PMC9730288
- DOI: 10.3389/fimmu.2022.931876
Modulating macrophage function to reinforce host innate resistance against Mycobacterium avium complex infection
Abstract
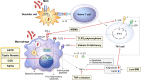
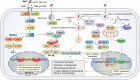
Mycobacterium avium complex (MAC) is the main causative agent of infectious diseases in humans among nontuberculous mycobacteria (NTM) that are ubiquitous organisms found in environmental media such as soil as well as in domestic and natural waters. MAC is a primary causative agent of NTM-lung disease that threaten immunocompromised or structural lung disease patients. The incidence and the prevalence of M. tuberculosis infection have been reduced, while MAC infections and mortality rates have increased, making it a cause of global health concern. The emergence of drug resistance and the side effects of long-term drug use have led to a poor outcome of treatment regimens against MAC infections. Therefore, the development of host-directed therapy (HDT) has recently gained interest, aiming to accelerate mycobacterial clearance and reversing lung damage by employing the immune system using a novel adjuvant strategy to improve the clinical outcome of MAC infection. Therefore, in this review, we discuss the innate immune responses that contribute to MAC infection focusing on macrophages, chief innate immune cells, and host susceptibility factors in patients. We also discuss potential HDTs that can act on the signaling pathway of macrophages, thereby contributing to antimycobacterial activity as a part of the innate immune response during MAC infection. Furthermore, this review provides new insights into MAC infection control that modulates and enhances macrophage function, promoting host antimicrobial activity in response to potential HDTs and thus presenting a deeper understanding of the interactions between macrophages and MACs during infection.
Keywords: Mycobacterium avium complex; host-directed therapy; innate immunity; macrophage; nontuberculous mycobacteria.
Copyright © 2022 Park, Lee, Choi, Jung, Shin and Shin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Recent advances in immunopathogenesis and clinical practice: mastering the challenge-managing of non-tuberculous mycobacteria.Front Immunol. 2025 Mar 19;16:1554544. doi: 10.3389/fimmu.2025.1554544. eCollection 2025. Front Immunol. 2025. PMID: 40176807 Free PMC article. Review.
-
[Strategies for Mycobacterium avium complex infection control in Japan: how do they improve the present situation?].Kekkaku. 2013 Mar;88(3):355-71. Kekkaku. 2013. PMID: 23672176 Japanese.
-
Reduced phagocytic activity of human alveolar macrophages infected with Mycobacterium avium complex.J Infect Chemother. 2022 Nov;28(11):1506-1512. doi: 10.1016/j.jiac.2022.07.018. Epub 2022 Aug 1. J Infect Chemother. 2022. PMID: 35926765
-
The New Frontier of Host-Directed Therapies for Mycobacterium avium Complex.Front Immunol. 2021 Jan 22;11:623119. doi: 10.3389/fimmu.2020.623119. eCollection 2020. Front Immunol. 2021. PMID: 33552087 Free PMC article. Review.
-
[Non-tuberculous mycobacteriosis. What has been coming out].Kekkaku. 2011 Feb;86(2):113-25. Kekkaku. 2011. PMID: 21404655 Japanese.
Cited by
-
Predictive risk factors of treatment-refractory Mycobacterium avium complex lung disease: a single-center retrospective cohort study.Ther Adv Infect Dis. 2025 Apr 11;12:20499361251331676. doi: 10.1177/20499361251331676. eCollection 2025 Jan-Dec. Ther Adv Infect Dis. 2025. PMID: 40292088 Free PMC article.
-
Intracellular iron accumulation facilitates mycobacterial infection in old mouse macrophages.Geroscience. 2024 Apr;46(2):2739-2754. doi: 10.1007/s11357-023-01048-1. Epub 2023 Dec 30. Geroscience. 2024. PMID: 38159133 Free PMC article.
-
Immunologic features of nontuberculous mycobacterial pulmonary disease based on spatially resolved whole transcriptomics.BMC Pulm Med. 2024 Aug 13;24(1):392. doi: 10.1186/s12890-024-03207-2. BMC Pulm Med. 2024. PMID: 39138424 Free PMC article.
-
Recent advances in immunopathogenesis and clinical practice: mastering the challenge-managing of non-tuberculous mycobacteria.Front Immunol. 2025 Mar 19;16:1554544. doi: 10.3389/fimmu.2025.1554544. eCollection 2025. Front Immunol. 2025. PMID: 40176807 Free PMC article. Review.
-
RNA-sequencing studies suggest that microRNAs and alternative splicing of pre-mRNAs modulate immune and inflammatory responses in Holstein cattle infected with Mycobacterium avium subsp. paratuberculosis.Front Immunol. 2025 Jun 25;16:1597736. doi: 10.3389/fimmu.2025.1597736. eCollection 2025. Front Immunol. 2025. PMID: 40636120 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

