Cellular immune states in SARS-CoV-2-induced disease
- PMID: 36505442
- PMCID: PMC9726761
- DOI: 10.3389/fimmu.2022.1016304
Cellular immune states in SARS-CoV-2-induced disease
Abstract
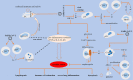
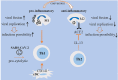
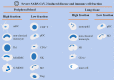
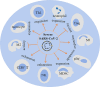
The general immune state plays important roles against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Cells of the immune system are encountering rapid changes during the acute phase of SARS-CoV-2-induced disease. Reduced fraction of functional CD8+ T cells, disrupted cross-talking between CD8+ T cells with dendritic cells (DCs), and impaired immunological T-cell memory, along with the higher presence of hyperactive neutrophils, high expansion of myeloid-derived suppressor cells (MDSCs) and non-classical monocytes, and attenuated cytotoxic capacity of natural killer (NK) cells, are all indicative of low efficient immunity against viral surge within the body. Immune state and responses from pro- or anti-inflammatory cells of the immune system to SARS-CoV-2 are discussed in this review. We also suggest some strategies to enhance the power of immune system against SARS-CoV-2-induced disease.
Keywords: CD8+ T cell; SARS-CoV-2; dendritic cell (DC); immunity; monocyte; myeloid-derived suppressor cell (MDSC); neutrophil; regulatory T cell (Treg).
Copyright © 2022 Mortezaee and Majidpoor.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Hammoudeh SM, Hammoudeh AM, Bhamidimarri PM, Al Safar H, Mahboub B, Künstner A, et al. . Systems immunology analysis reveals the contribution of pulmonary and extrapulmonary tissues to the immunopathogenesis of severe COVID-19 patients. Front Immunol (2021) 12. doi: 10.3389/fimmu.2021.595150 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

