Sex bias in lymphocytes: Implications for autoimmune diseases
- PMID: 36505451
- PMCID: PMC9730535
- DOI: 10.3389/fimmu.2022.945762
Sex bias in lymphocytes: Implications for autoimmune diseases
Abstract
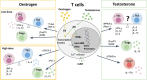
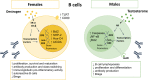
Autoimmune diseases are characterized by a significant sex dimorphism, with women showing increased susceptibility to disease. This is, at least in part, due to sex-dependent differences in the immune system that are influenced by the complex interplay between sex hormones and sex chromosomes, with contribution from sociological factors, diet and gut microbiota. Sex differences are evident in the number and function of lymphocyte populations. Women mount a stronger pro-inflammatory response than males, with increased lymphocyte proliferation, activation and pro-inflammatory cytokine production, whereas men display expanded regulatory cell subsets. Ageing alters the immune landscape of men and women in differing ways, resulting in changes in autoimmune disease susceptibility. Here we review the current literature on sex differences in lymphocyte function, the factors that influence this, and the implications for autoimmune disease. We propose that improved understanding of sex bias in lymphocyte function can provide sex-specific tailoring of treatment strategies for better management of autoimmune diseases.
Keywords: B cells; T cells; estrogen; lymphocytes; sex; sex-bias; testosterone.
Copyright © 2022 Dodd and Menon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Connect Immune Research . Are you autimmune aware? report for parlimentarians into autoimmune conditions (2018). Available at: https://www.immunology.org/sites/default/files/connect-immune-research-a....
-
- Rindfleisch JA, Muller D. Diagnosis and management of rheumatoid arthritis. Am Fam Phys (2005) 72:1037–47. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

