Nasal administration of anti-CD3 monoclonal antibody modulates effector CD8+ T cell function and induces a regulatory response in T cells in human subjects
- PMID: 36505477
- PMCID: PMC9727230
- DOI: 10.3389/fimmu.2022.956907
Nasal administration of anti-CD3 monoclonal antibody modulates effector CD8+ T cell function and induces a regulatory response in T cells in human subjects
Abstract
Background: Parenteral anti-CD3 Mab (OKT3) has been used to treat transplant rejection and parental administration of a humanized anti-CD3 Mab (Teplizumab) showed positive effects in diabetes. Nasal administration of anti-CD3 Mab has not been carried out in humans. Nasal anti-CD3 Mab suppresses autoimmune diseases and central nervous system (CNS) inflammation in animal models. We investigated the safety and immune effects of a fully humanized, previously uncharacterized nasal anti-CD3 Mab (Foralumab) in humans and its in vitro stimulatory properties.
Methods: In vitro, Foralumab were compared to UCHT1 anti-human CD3 mAb. For human administration, 27 healthy volunteers (9 per group) received nasal Foralumab or placebo at a dose of 10ug, 50ug, or 250ug daily for 5 days. Safety was assessed and immune parameters measured on day 1 (pre-treatment), 7, 14, and 30 by FACS and by scRNAseq.
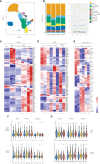
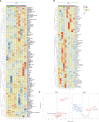
Results: In vitro, Foralumab preferentially induced CD8+ T cell stimulation, reduced CD4+ T cell proliferation and lowered expression of IFNg, IL-17 and TNFa. Foralumab induced LAP, TIGIT, and KLRG1 immune checkpoint molecules on CD8+ and CD4+ T cells in a mechanism independent of CD8 T cells. In vivo, nasal Foralumab did not modulate CD3 from the T cell surface at any dose. Immune effects were primarily observed at the 50ug dose and consisted of reduction of CD8+ effector memory cells, an increase in naive CD8+ and CD4+ T cells, and reduced CD8+ T cell granzyme B and perforin expression. Differentially expressed genes observed by scRNAseq in CD8+ and CD4+ populations promoted survival and were anti-inflammatory. In the CD8+ TEMRA population there was induction of TIGIT, TGFB1 and KIR3DL2, indicative of a regulatory phenotype. In the memory CD4+ population, there was induction of CTLA4, KLRG1, and TGFB whereas there was an induction of TGF-B1 in naïve CD4+ T cells. In monocytes, there was induction of genes (HLA-DP, HLA-DQ) that promote a less inflammatory immune response. No side effects were observed, and no subjects developed human anti-mouse antibodies.
Conclusion: These findings demonstrate that nasal Foralumab is safe and immunologically active in humans and presents a new avenue for the treatment of autoimmune and CNS diseases.
Keywords: CD8+ T cells; Foralumab; Tregs; immunomodulation; nasal anti-CD3.
Copyright © 2022 Chitnis, Kaskow, Case, Hanus, Li, Varghese, Healy, Gauthier, Saraceno, Saxena, Lokhande, Moreira, Zurawski, Roditi, Bergmark, Giovannoni, Torti, Li, Quintana, Clementi, Shailubhai, Weiner and Baecher-Allan.
Conflict of interest statement
WC was employed by Clementi, Ltd. HLW is chair of the scientific advisory board of Tiziana Life Sciences and received consulting fees and stock options from the company. KS is an employee of Tiziana Life Sciences. TC is a member of the scientific advisory board and serves as a consultant to Tiziana Life Sciences. CMB-A serves as a consultant to Tiziana Life Sciences. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

