Plasticity impairment alters community structure but permits successful pattern separation in a hippocampal network model
- PMID: 36505514
- PMCID: PMC9729278
- DOI: 10.3389/fncel.2022.977769
Plasticity impairment alters community structure but permits successful pattern separation in a hippocampal network model
Abstract
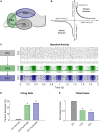
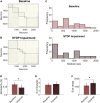
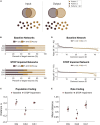
Patients who suffer from traumatic brain injury (TBI) often complain of learning and memory problems. Their symptoms are principally mediated by the hippocampus and the ability to adapt to stimulus, also known as neural plasticity. Therefore, one plausible injury mechanism is plasticity impairment, which currently lacks comprehensive investigation across TBI research. For these studies, we used a computational network model of the hippocampus that includes the dentate gyrus, CA3, and CA1 with neuron-scale resolution. We simulated mild injury through weakened spike-timing-dependent plasticity (STDP), which modulates synaptic weights according to causal spike timing. In preliminary work, we found functional deficits consisting of decreased firing rate and broadband power in areas CA3 and CA1 after STDP impairment. To address structural changes with these studies, we applied modularity analysis to evaluate how STDP impairment modifies community structure in the hippocampal network. We also studied the emergent function of network-based learning and found that impaired networks could acquire conditioned responses after training, but the magnitude of the response was significantly lower. Furthermore, we examined pattern separation, a prerequisite of learning, by entraining two overlapping patterns. Contrary to our initial hypothesis, impaired networks did not exhibit deficits in pattern separation with either population- or rate-based coding. Collectively, these results demonstrate how a mechanism of injury that operates at the synapse regulates circuit function.
Keywords: cellular networks; memory impairment; patterned structure; plasticity; traumatic brain injury.
Copyright © 2022 Schumm, Gabrieli and Meaney.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Plasticity impairment exposes CA3 vulnerability in a hippocampal network model of mild traumatic brain injury.Hippocampus. 2022 Mar;32(3):231-250. doi: 10.1002/hipo.23402. Epub 2022 Jan 3. Hippocampus. 2022. PMID: 34978378
-
The race to learn: spike timing and STDP can coordinate learning and recall in CA3.Hippocampus. 2011 Jun;21(6):647-60. doi: 10.1002/hipo.20777. Epub 2010 Mar 15. Hippocampus. 2011. PMID: 20232384
-
GABAA receptor-mediated feedforward and feedback inhibition differentially modulate hippocampal spike timing-dependent plasticity.Biochem Biophys Res Commun. 2012 Oct 26;427(3):466-72. doi: 10.1016/j.bbrc.2012.08.081. Epub 2012 Aug 24. Biochem Biophys Res Commun. 2012. PMID: 22940549
-
Voltage Imaging in the Study of Hippocampal Circuit Function and Plasticity.Adv Exp Med Biol. 2015;859:197-211. doi: 10.1007/978-3-319-17641-3_8. Adv Exp Med Biol. 2015. PMID: 26238054 Review.
-
A computational theory of hippocampal function, and tests of the theory: new developments.Neurosci Biobehav Rev. 2015 Jan;48:92-147. doi: 10.1016/j.neubiorev.2014.11.009. Epub 2014 Nov 20. Neurosci Biobehav Rev. 2015. PMID: 25446947 Review.
Cited by
-
Morphometric and microstructural characteristics of hippocampal subfields in mesial temporal lobe epilepsy and their correlates with mnemonic discrimination.Front Neurol. 2023 Feb 14;14:1096873. doi: 10.3389/fneur.2023.1096873. eCollection 2023. Front Neurol. 2023. PMID: 36864916 Free PMC article.
References
-
- Aungst S. L., Kabadi S. V., Thompson S. M., Stoica B. A., Faden A. I. (2014). Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J. Cereb. Blood Flow. Metab. 34 1223–1232. 10.1038/jcbfm.2014.75 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

