Ocular adverse effects of COVID-19 vaccines: A systematic review
- PMID: 36505575
- PMCID: PMC9731019
- DOI: 10.4103/jfmpc.jfmpc_747_22
Ocular adverse effects of COVID-19 vaccines: A systematic review
Abstract
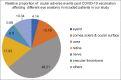
The COVID-19 pandemic has led to the development and rollout of several vaccines worldwide at unprecedented pace. This systematic review of published literature has been undertaken to spread awareness among general physicians and ophthalmologists about the various reported adverse effects in the eye following COVID-19 vaccination. A systematic search was performed on 25 January 2022 through PuBMed, Medline and Google scholar for publications on ocular adverse effects after COVID-19 vaccination. One brief communication, four retrospective case series, sixteen case reports, and five letters to editors were included. Ocular manifestations most commonly appear in the uvea and retina. Other manifestations are seen on the eyelid, cornea and ocular surface, and in cranial nerves innervating the eye. The incidence rate of these manifestations is quite low after COVID-19 vaccinations. Our systematic review meticulously enumerates various adverse effects of COVID -19 vaccine on the eye. Most of these adverse effects are transient and observed to resolve without any sequelae except for cases of retinal and ophthalmic vascular occlusions and corneal graft rejections. An emphasis on close follow-up and a need to delay vaccination and modified therapy to control flare up of signs and symptoms in certain sub-populations, Graves' disease (autoimmune etiology), pre-existing uveal inflammation and corneal graft cases are warranted. We need long-term, larger, multicentric studies to substantiate our findings and establish the causal relationship with certainty. Mass vaccinations to curb this pandemic after outweighing the ocular risks associated with it is warranted.
Keywords: COVID-19; Cornea; SARS-COV-2; coronavirus; eye; eyelid; neuropathy; ocular adverse effects; retinopathy; uvea; vaccination.
Copyright: © 2022 Journal of Family Medicine and Primary Care.
Conflict of interest statement
There are no conflicts of interest.
Figures



Similar articles
-
Ocular Manifestations after Receiving COVID-19 Vaccine: A Systematic Review.Vaccines (Basel). 2021 Nov 27;9(12):1404. doi: 10.3390/vaccines9121404. Vaccines (Basel). 2021. PMID: 34960150 Free PMC article. Review.
-
After the Storm: Ophthalmic Manifestations of COVID-19 Vaccines.Indian J Ophthalmol. 2021 Dec;69(12):3398-3420. doi: 10.4103/ijo.IJO_2824_21. Indian J Ophthalmol. 2021. PMID: 34826968 Free PMC article. Review.
-
Ocular Adverse Events After COVID-19 Vaccination.Ocul Immunol Inflamm. 2021 Aug 18;29(6):1216-1224. doi: 10.1080/09273948.2021.1976221. Epub 2021 Sep 24. Ocul Immunol Inflamm. 2021. PMID: 34559576 Free PMC article. Review.
-
[Ocular symptoms in SARS-CoV-2 infection].Pol Merkur Lekarski. 2022 Apr 19;50(296):86-93. Pol Merkur Lekarski. 2022. PMID: 35436269 Review. Polish.
-
Ocular Complications Following Vaccination for COVID-19: A One-Year Retrospective.Vaccines (Basel). 2022 Feb 21;10(2):342. doi: 10.3390/vaccines10020342. Vaccines (Basel). 2022. PMID: 35214800 Free PMC article. Review.
Cited by
-
Retinal Vein Occlusion Amongst People Vaccinated by mRNA- and Viral Vector- COVID-19 Vaccines: A Systematic Review.Clin Ophthalmol. 2023 Sep 28;17:2825-2842. doi: 10.2147/OPTH.S426428. eCollection 2023. Clin Ophthalmol. 2023. PMID: 37794952 Free PMC article. Review.
-
The Review of Ophthalmic Symptoms in COVID-19.Clin Ophthalmol. 2024 May 23;18:1417-1432. doi: 10.2147/OPTH.S460224. eCollection 2024. Clin Ophthalmol. 2024. PMID: 38803556 Free PMC article. Review.
-
COVID-19 vaccination and corneal allograft rejection- a review.Front Cell Infect Microbiol. 2023 Dec 15;13:1307655. doi: 10.3389/fcimb.2023.1307655. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38162575 Free PMC article. Review.
-
Manifestations of COVID-19 in the posterior eye segment - Up-to-date.Oman J Ophthalmol. 2024 Jun 27;17(2):166-172. doi: 10.4103/ojo.ojo_212_22. eCollection 2024 May-Aug. Oman J Ophthalmol. 2024. PMID: 39132129 Free PMC article. Review.
-
Ocular Complications after COVID-19 Vaccination: A Systematic Review.Medicina (Kaunas). 2024 Jan 31;60(2):249. doi: 10.3390/medicina60020249. Medicina (Kaunas). 2024. PMID: 38399537 Free PMC article.
References
-
- [Last accessed on 2022 Jan 25]. Available from: http://www.cdc.gov/coronavirus/2019ncov/vaccines/differentvaccines/Moder... .
-
- [Last accessed on 2022 Jan 25]. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand... .
-
- [Last accessed on 2022 Jan 25]. Available from: https://www.mohfw.gov.in/covid_vaccination/
-
- JAMA Network Open—Instructions for Authors: Ratings of the Quality of the Evidence. [Last accessed on 2022 Jan 25]. Available from: https://jamanetwork.com/journals/jamanetworkopen/pages/instructions-for-... .
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
