Loss of GABA co-transmission from cholinergic neurons impairs behaviors related to hippocampal, striatal, and medial prefrontal cortex functions
- PMID: 36505727
- PMCID: PMC9730538
- DOI: 10.3389/fnbeh.2022.1067409
Loss of GABA co-transmission from cholinergic neurons impairs behaviors related to hippocampal, striatal, and medial prefrontal cortex functions
Abstract
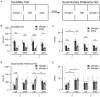
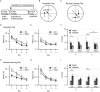
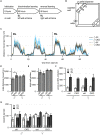
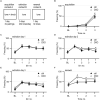
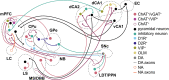
Introduction: Altered signaling or function of acetylcholine (ACh) has been reported in various neurological diseases, including Alzheimer's disease, Tourette syndrome, epilepsy among others. Many neurons that release ACh also co-transmit the neurotransmitter gamma-aminobutyrate (GABA) at synapses in the hippocampus, striatum, substantia nigra, and medial prefrontal cortex (mPFC). Although ACh transmission is crucial for higher brain functions such as learning and memory, the role of co-transmitted GABA from ACh neurons in brain function remains unknown. Thus, the overarching goal of this study was to investigate how a systemic loss of GABA co-transmission from ACh neurons affected the behavioral performance of mice. Methods: To do this, we used a conditional knock-out mouse of the vesicular GABA transporter (vGAT) crossed with the ChAT-Cre driver line to selectively ablate GABA co-transmission at ACh synapses. In a comprehensive series of standardized behavioral assays, we compared Cre-negative control mice with Cre-positive vGAT knock-out mice of both sexes. Results: Loss of GABA co-transmission from ACh neurons did not disrupt the animal's sociability, motor skills or sensation. However, in the absence of GABA co-transmission, we found significant alterations in social, spatial and fear memory as well as a reduced reliance on striatum-dependent response strategies in a T-maze. In addition, male conditional knockout (CKO) mice showed increased locomotion. Discussion: Taken together, the loss of GABA co-transmission leads to deficits in higher brain functions and behaviors. Therefore, we propose that ACh/GABA co-transmission modulates neural circuitry involved in the affected behaviors.
Keywords: GABA; acetylcholine; animal behavior; co-transmission; transgenic mice.
Copyright © 2022 Goral, Harper, Bernstein, Fry, Lamb, Moy, Cushman and Yakel.
Figures

Similar articles
-
Developmental regulation of GABAergic gene expression in forebrain cholinergic neurons.Front Neural Circuits. 2023 Mar 24;17:1125071. doi: 10.3389/fncir.2023.1125071. eCollection 2023. Front Neural Circuits. 2023. PMID: 37035505 Free PMC article.
-
Reduced striatal acetylcholine efflux in the R6/2 mouse model of Huntington's disease: an examination of the role of altered inhibitory and excitatory mechanisms.Exp Neurol. 2011 Dec;232(2):119-25. doi: 10.1016/j.expneurol.2011.08.010. Epub 2011 Aug 16. Exp Neurol. 2011. PMID: 21864528
-
Impairment of reward-related learning by cholinergic cell ablation in the striatum.Proc Natl Acad Sci U S A. 2003 Jun 24;100(13):7965-70. doi: 10.1073/pnas.1032899100. Epub 2003 Jun 11. Proc Natl Acad Sci U S A. 2003. PMID: 12802017 Free PMC article.
-
Cholinergic circuits in cognitive flexibility.Neuroscience. 2017 Mar 14;345:130-141. doi: 10.1016/j.neuroscience.2016.09.013. Epub 2016 Sep 15. Neuroscience. 2017. PMID: 27641830 Review.
-
Illuminating the role of cholinergic signaling in circuits of attention and emotionally salient behaviors.Front Synaptic Neurosci. 2014 Oct 27;6:24. doi: 10.3389/fnsyn.2014.00024. eCollection 2014. Front Synaptic Neurosci. 2014. PMID: 25386136 Free PMC article. Review.
Cited by
-
Developmental regulation of GABAergic gene expression in forebrain cholinergic neurons.Front Neural Circuits. 2023 Mar 24;17:1125071. doi: 10.3389/fncir.2023.1125071. eCollection 2023. Front Neural Circuits. 2023. PMID: 37035505 Free PMC article.
-
Acetylcholine Neurons Become Cholinergic during Three Time Windows in the Developing Mouse Brain.eNeuro. 2024 Jul 16;11(7):ENEURO.0542-23.2024. doi: 10.1523/ENEURO.0542-23.2024. Print 2024 Jul. eNeuro. 2024. PMID: 38942474 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

